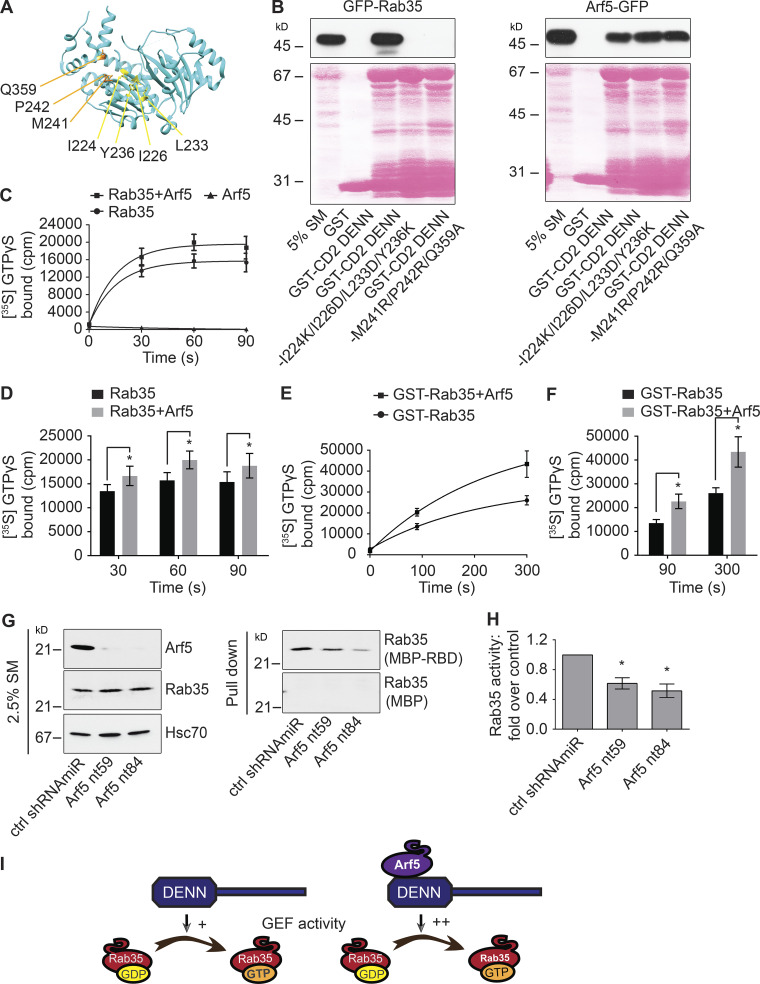
Figure 4.
Arf5 activates connecdenn GEF activity toward Rab35. (A) A ribbon representation of the structure of the DENN domain of connecdenn 2 (DENND1B) taken from Wu et al. (2011; Protein Data Bank ID 3TW8). Amino acids required for interaction with and GEF activity toward Rab35 are indicated. (B) Detergent-soluble lysates were prepared from HEK-293 cells transfected with GFP-Rab35 or Arf5-GFP as indicated and incubated with GST alone, GST-connecdenn 2 (CD2) DENN domain, or the CD2 DENN domain with the quadrupole or triple point mutations indicated. Glutathione-Sepharose beads were washed, and the bound proteins were prepared for immunoblot with an antibody recognizing GFP. An aliquot of the cell lysate equal to 5% of that added to the beads was run as a starting material (SM). The Ponceau-stained transfer indicates the levels of fusion protein. The migration of molecular mass markers (in kD) is indicated. (C) In vitro GEF assays using purified connecdenn 1 (CD1) DENN domain and different combinations of Rab35 and Arf5 as indicated. The amount of [35S]GTPγS transferred to the GTPases was determined by collecting the reactions on filters, followed by scintillation counting. The relative incorporation of [35S]GTPγS is plotted over time; data represent mean ± SEM; n = 8. The curve was fitted by nonlinear regression one-phase association. (D) Quantification of the amount of [35S]GTPγS transferred to Rab35 at 30, 60, and 90 s from experiments as in C. Statistical analysis employed a two-way ANOVA followed by Bonferroni’s multiple comparisons test. *, P < 0.05; n = 8. (E) In vitro GEF assays as described in C except using GST-tagged Rab35 coupled to glutathione-Sepharose beads in the presence or absence of purified Arf5, as indicated. The amount of [35S]GTPγS transferred to Rab35 was determined by pelleting the beads in a microfuge. The relative incorporation of [35S]GTPγS is plotted over time; data represent mean ± SEM; n = 4. The curve was fitted by nonlinear regression one-phase association. (F) Quantification of the amount of [35S]GTPγS transferred to Rab35 at 90 and 300 s from experiments as in E. Data are shown as mean ± SEM. Statistical analysis employed a two-way ANOVA followed by Bonferroni’s multiple comparisons test. *, P < 0.05; n = 4. (G) Soluble lysates were prepared from HEK-293T cells transduced with control (ctrl) shRNAmiR or two different shRNAmiRs targeting Arf5 as indicated. The lysates were incubated with either MBP alone or MBP conjugated to the RBD of RUSC2 (MBP-RBD), with the MBP proteins prebound to amylose-Sepharose beads. Beads were washed, and the bound proteins were processed for immunoblot with antibody specific for Rab35 (pull-down). An aliquot of the cell lysate (starting material [SM]) equal to 2.5% of that added to the beads was run in parallel and immunblotted with antibodies specific for the indicated proteins. The migration of molecular mass markers (in kD) is indicated. (H) Quantification of the amount of Rab35 bound to the MBP-RBD from the Arf5 knockdown cells normalized to that from the control shRNAmiR-treated cells as in G. Data are shown as mean ± SEM. Statistical analysis employed a one-way ANOVA followed by Dunnett’s post hoc test. *, P < 0.05; n = 5. (I) Schematic model showing that Arf5 interacts with the DENN domain of connecdenn 1/2 to stimulate its GEF activity toward Rab35.