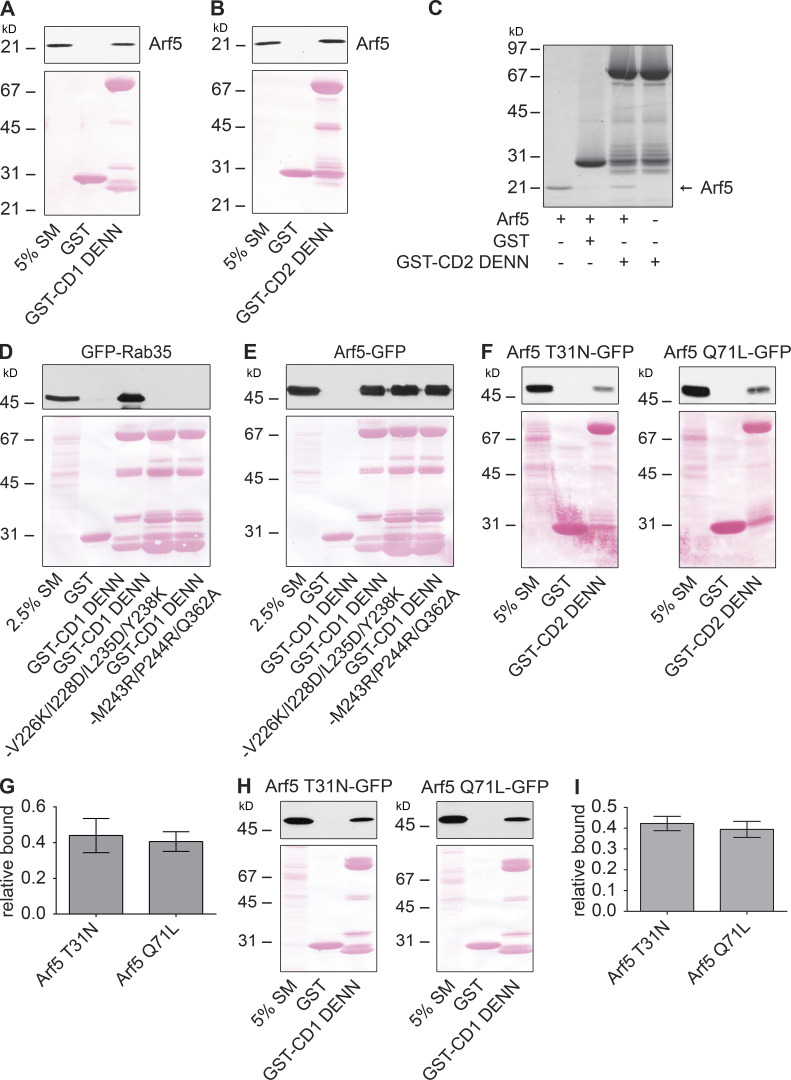
Figure S1.
Arf5 binds directly to connecdenn DENN domain. (A and B) Arf5-GST was purified from bacteria, and the GST tag was removed by proteolytic cleavage. Purified Arf5 was incubated with GST or GST-DENN domain of connecdenn 1 (CD1; A) or 2 (CD2; B) prebound to glutathione-Sepharose. The glutathione-Sepharose beads were washed, and bound proteins were prepared for immunoblot with an Arf5 antibody. An aliquot of purified Arf5 equal to 5% of that added to the beads was run as a starting material (SM). The Ponceau-stained transfer indicates the levels of fusion protein. The migration of a molecular mass markers (in kD) is indicated. (C) Samples prepared and processed as in B but analyzed with a Coomassie-stained gel. Arrow denotes the purified Arf5. The migration of molecular mass markers (in kD) is indicated. (D and E) Detergent-soluble lysates were prepared from HEK-293T cells transfected with GFP-Rab35 (D) or Arf5-GFP (E) and incubated with GST alone, GST–connecdenn 1 (CD1) DENN domain, or the CD1 DENN domain with the quadrupole or triple point mutations indicated. Glutathione-Sepharose beads were washed, and the bound proteins were prepared for immunoblot with an antibody recognizing GFP. An aliquot of the cell lysate equal to 2.5% of that added to the beads was run as a starting material (SM). The Ponceau-stained transfer indicates the levels of fusion protein. The migration of molecular mass markers (in kD) is indicated. (F) Detergent-soluble lysate prepared from HEK-293T cells transfected with Arf5 mutants with C-terminal GFP tags (T31N, GDP-bound; Q71L, GTP-bound) were incubated with GST or GST conjugated to the DENN domain of connecdenn 2 (CD2), prebound to glutathione-Sepharose. The glutathione-Sepharose beads were washed, and bound proteins were prepared for immunoblot with an antibody recognizing GFP. An aliquot of the cell lysates equal to 5% of that added to the beads was run as a starting material (SM). The Ponceau-stained transfer indicates the levels of fusion protein. The migration of molecular mass markers (in kD) is indicated. (G) Quantification of Arf5 bound from experiments as in F. Data are shown as mean ± SEM. Statistical analysis employed an unpaired t test; n = 5. (H) Detergent-soluble lysate prepared from HEK-293T cells transfected with Arf5 mutants as described in F were incubated with GST or GST conjugated to the DENN domain of connecdenn 1 (CD1) prebound to glutathione-Sepharose. The glutathione-Sepharose beads were washed, and bound proteins were prepared for immunoblot with an antibody recognizing GFP. An aliquot of the cell lysates equal to 5% of that added to the beads was run as a starting material (SM). The Ponceau-stained transfer indicates the levels of fusion protein. The migration of molecular mass markers (in kD) is indicated. (I) Quantification of Arf5 bound from experiments as in H. Data are shown as mean ± SEM. Statistical analysis employed an unpaired t test; n = 3.