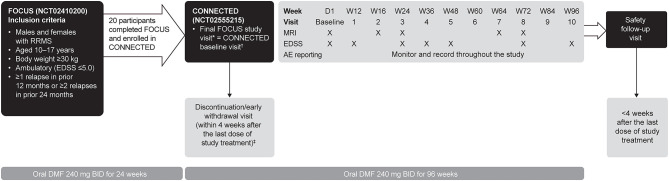
Figure 1.
CONNECTED study design. AE, adverse event; BID, twice daily; D, day; DMF, delayed-release dimethyl fumarate; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; RRMS, relapsing-remitting multiple sclerosis; W, week. *Eligibility for CONNECTED was determined at the final study visit in FOCUS or within 4 weeks before CONNECTED study entry. †Within 4 weeks of the final study visit for FOCUS, if the two visits could not have been held at the same time. ‡Participants who discontinued treatment early could remain in the study and continue protocol-required tests and assessments, and those who withdrew prematurely were encouraged to complete the safety follow-up visit.