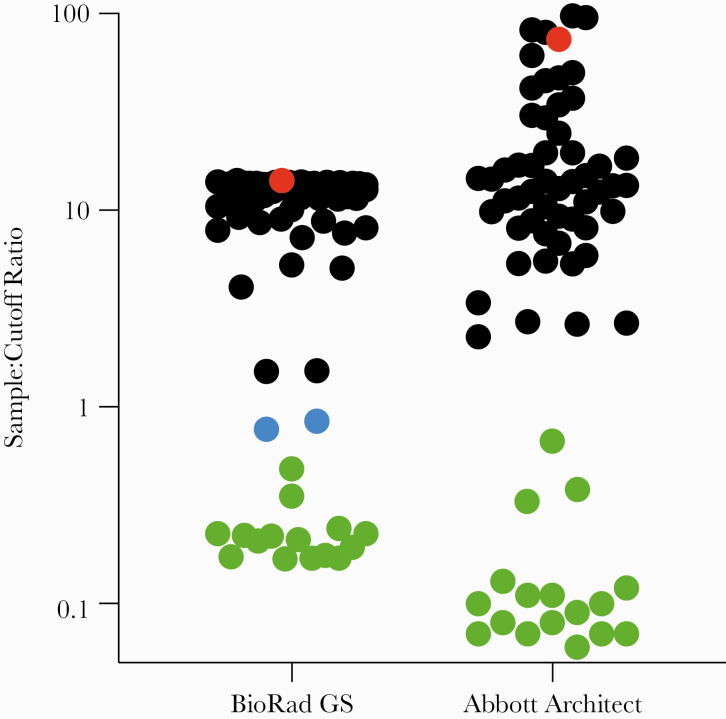
Figure 1.
Assessment of fourth-generation human immunodeficiency virus (HIV) tests compared with OraQuick ADVANCE. End-of-study plasma samples from participants in the HIV Vaccine Trial Network (HVTN) 117/HPX2004 study were assayed for vaccine-induced seropositivity using 2 routine fourth-generation HIV tests. Sample/cutoff ratios are given: nonreactive samples are green, samples reported as equivocal are blue, reactive samples are black except for the lone sample that was reactive by OraQuick ADVANCE, which is red.