Figure 2: Ligand-specific signaling through EGFR homodimers.
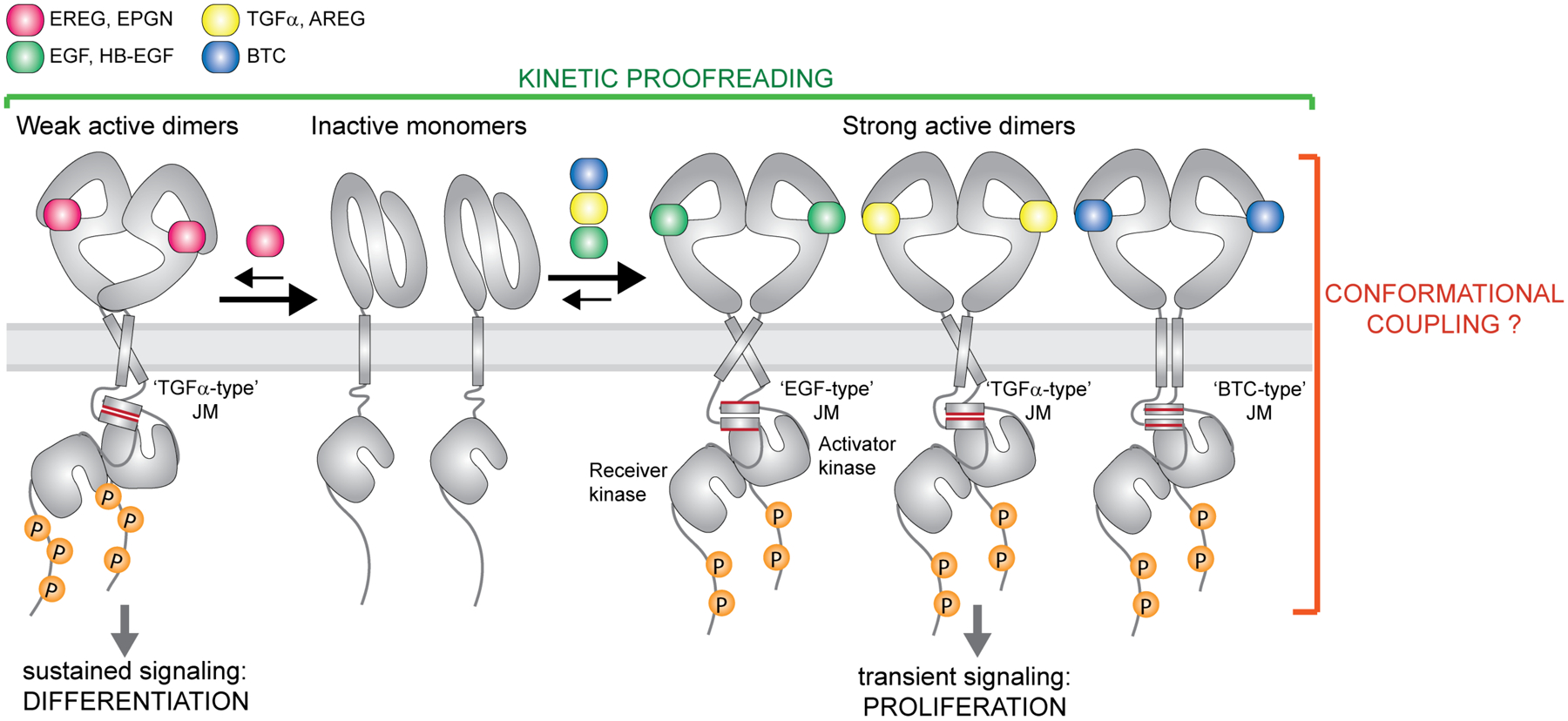
EGFR forms high-affinity complexes with its cognate ligands Transforming Growth Factor α (TGFα), Epidermal Growth Factor (EGF), Amphiregulin (AREG), Betacellulin (BTC) and Heparin-Binding-EGF (HB-EGF) but lower-affinity complexes with Epiregulin (EREG) and Epigen (EPGN). Available structures of EGF, TGFα±EPGN and EREG bound to EGFR ECDs are so far consistent with the EGFR ECDs adopting symmetric dimers with high affinity ligands and asymmetric structures with lower affinity ligands. Weaker EGFR dimers lead to sustained signaling and cell differentiation while formation of strong complexes causes transient receptor signaling and cell proliferation. Thus, the dynamics of ligand-dependent receptor association and dissociation has an important impact on the recruitment of downstream effectors and the ligand-specific cellular responses (kinetic proofreading). Biochemical studies on the EGFR juxtamembrane (JM) segment and studies of full-length EGFR in cells are consistent with the presence of the JM coiled-coil dimer in active receptor complex. Conformation of the JM dimer appears to change depending on a bound ligand. At least three specific JM dimer modes have been described for EGFR, denoted here as ‘TGFα-type’, the ‘EGF-type’ and the ‘BTC-type’.
