Figure 4: Co-receptors assure ligand specificity and control of downstream signaling in the FGF and RET signaling systems.
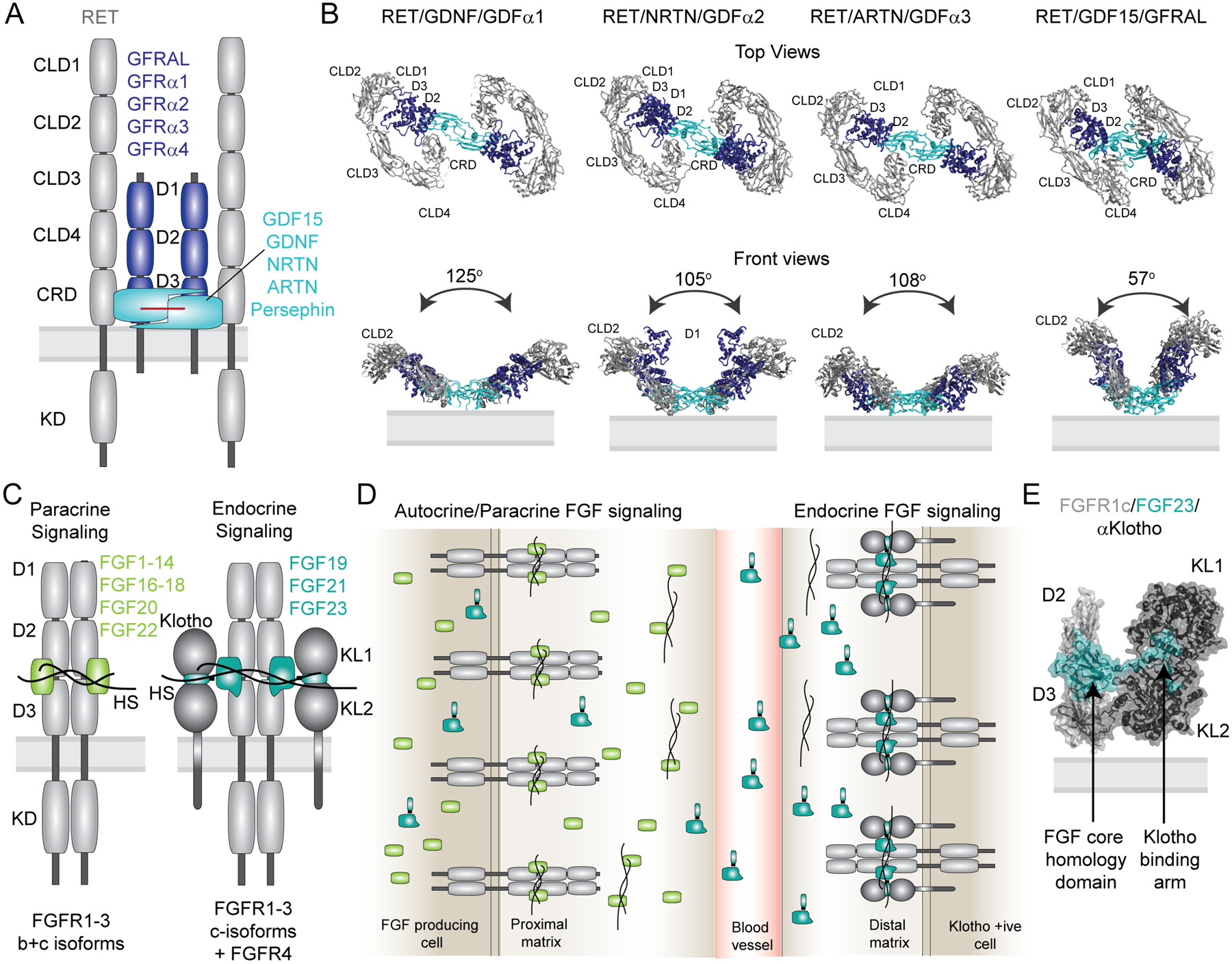
(A) Domain architecture of the 2/2/2 RET receptor/co-receptor/ligand complex. The receptor with its structural domains is shown in grey. The co-receptors GFRAL and GFRα1–4 are shown in blue and the ligands GDF15, GDNF, NRTN, ARTN, Persephin are shown in cyan. (B) Cryo-EM structures of the 2:2:2 RET ECD receptor/co-receptor/ligand complexes are shown as top and front views (PDB codes from left to right: 6Q2N, 6Q2O, 6Q2S, 6Q2J). Adapted from [113]. (C) Domain architecture of the autocrine/paracrine and endocrine active 2:2 and 2:2:2 complexes of receptor/ligand and receptor/ligand/Klotho complexes, respectively. Both representations illustrate heparan sulfate (HS) bound to the complexes. (D) Simplified overview of receptor specificity for autocrine/paracrine and endocrine ligands. Paracrine/autocrine ligands bind to HS with high affinity, which traps the ligands close to the site of secretion and enables them to bind the cognate receptors. Autocrine/paracrine ligands can be either specific towards FGFR1–3b or c isoforms or bind both isoforms promiscuously. The presence of HS is mandatory for activation by the paracrine/autocrine ligands. Endocrine ligands FGF19, FGF21 and FGF23 have low affinity for both FGFR isoforms and HS and require the presence of either α-Klotho or β-Klotho co-receptors for binding and activation of their cognate FGF receptors. The low affinity of endocrine ligands to HS facilitates secretion from the tissue of origin to distal organs that express the respective Klotho/FGFRc complexes. Despite low affinity in the absence of Klotho, HS appears to be required for receptor dimerization upon ligand binding. (E) Crystal structure of the FGFR1c/α-Klotho/FGF23 ECD complex (PDB code: 5W21). The structure illustrates tight interactions between FGF23 and the α-Klotho ECD via the Klotho binding arm, and with the receptor ECD via a truncated FGF core homology domain.
