Abstract
A 65-year-old man, a smoker, presented to the emergency department with progressive digital ischaemia, fever and weight loss. The clinical examination revealed generalised lymphadenopathy and ischaemic changes of the right distal phalanges of the second, third and fourth fingers. He had an ultrasound-guided biopsy of the cervical lymph node, which showed histopathological findings of classic Hodgkin’s lymphoma. Paraneoplastic acral vascular syndrome (PAVS) is a rare phenomenon and seen more in solid malignancies. There are very few reported cases of PAVS in haematological malignancies, including Hodgkin’s lymphoma. This case highlights the idea that the presence of acral vascular syndrome—especially in older patients—should alert physicians to search for an underlying malignancy as part of the medical evaluation. Also, it shows that medical treatment may slow the progress of the digital ischaemia until the culprit tumour has been identified and treated.
Keywords: haematology (incl blood transfusion), vasculitis
Background
Paraneoplastic acral vascular syndrome (PAVS) is a rare phenomenon associated with various malignancies, especially adenocarcinomas.1 The reported incidence of PAVS is two cases per 100 000 patients per year.2 There are very few cases of PAVS reported in patients with Hodgkin’s lymphoma.3–6 The underlying pathophysiology of PAVS is not fully understood but multiple factors such as a vaso-occlusive process, an immune complex-mediated reaction and impaired haemostasis might be involved. Among the previously reported cases, various medical and surgical interventions were attempted but the outcomes varied among the cases. However, the treatment of the underlying malignancy was associated with a remission of the digital symptoms in most cases.2 7 8
Case presentation
A 65-year-old man, a smoker, presented to the emergency department at Sultan Qaboos University Hospital (SQUH) with a 10-day history of progressive painful, bluish-black discolouration of his right-hand fingers (second, third and fourth fingers) which had been preceded by a 1-month history of intermittent fever, malaise and weight loss. The patient was not known to have any medical conditions, and he took no regular medications. Moreover, he denied any recent travel history and drug or alcohol misuse.
The patient had previously been evaluated in a local hospital regarding his digital complaint. His work-up had revealed a normal ECG, unremarkable transthoracic echocardiogram (ECHO) findings and normal upper limb arterial duplex. He had an excisional biopsy of the cervical lymph node, which showed reactive lymphadenopathy. The patient had been started on warfarin as an anticoagulant to treat his digital ischaemia presumed secondary to systemic embolisation. The patient then presented to SQUH for a second medical opinion.
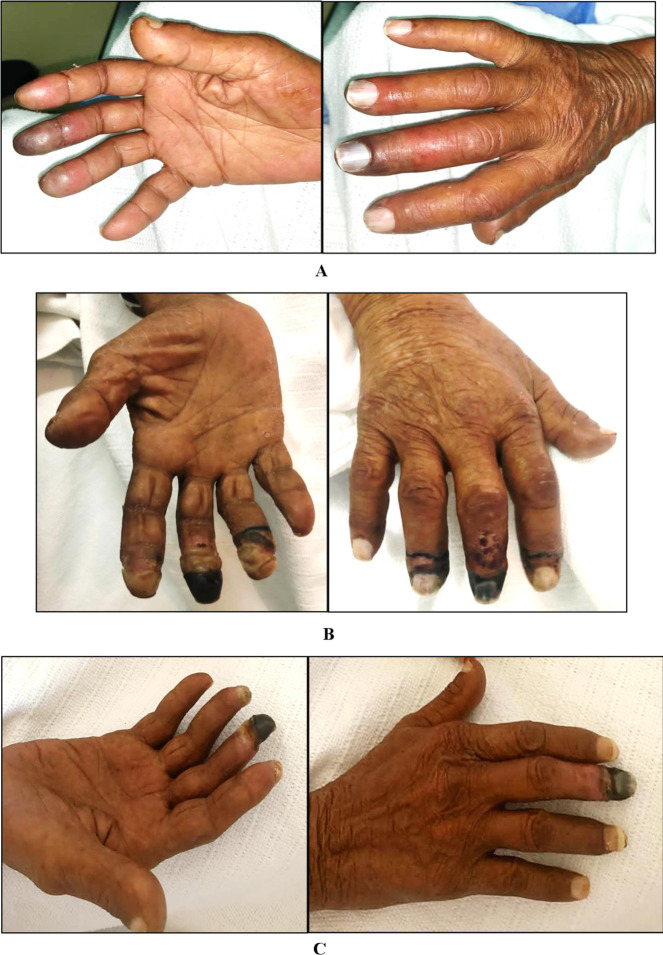
On physical examination, he appeared well, alert and oriented. He was mildly dehydrated but not pale or jaundiced. His vitals were as follows: temperature 36.9°C, blood pressure 128/70 mm Hg, heart rate 80 beats/min and oxygen saturation 99% on ambient air. The upper limb examination revealed multiple tender digital cyanotic and gangrenous changes involving the right distal phalanges (second, third and fourth) (figure 1A). All peripheral pulses were palpable, and the lower limb examination was unremarkable. His sensory and motor neurological examinations were normal. He had generalised lymphadenopathy of variable sizes characterised by firm, rounded, mobile and non-tender lymph nodes. The cardiac auscultation revealed a grade 2/6 pan systolic murmur at the apex. There were no stigmata of infective endocarditis (IE) and the remainder of the physical examination was unremarkable.
Figure 1.
Interval improvement of digital ischaemia. (A) At presentation. (B) After medical therapy. (C) After chemotherapy.
Investigations
The important laboratory findings are presented in table 1. As summarised, the patient had anaemia with raised inflammatory markers. Due to warfarin, he had a deranged coagulation profile which was normalised after vitamin K administration. Other investigations, including blood film, rheumatoid factor, antinuclear antibody, cyclic citrullinated peptide antibody, antineutrophil cytoplasmic antibodies, antiphospholipid antibodies, cryoglobulin, lupus anticoagulant, complement levels, extractable nuclear antigens, serum and protein electrophoresis, serum paraneoplastic screen, three sets of blood cultures, Q fever serology, Brucella serology, urea and electrolytes, liver function test, bone profile and uric acid level, were unremarkable.
Table 1.
Summary of the initial laboratory tests results
| Test | Result | Normal range |
| Hb (g/L) | 79 | 115-155 |
| Haematocrit (L/L) | 0.22 | 0.350–0.450 |
| MCV (fL) | 76.2 | 78.0–96.0 |
| MCH (pg) | 24.4 | 26.0–33.0 |
| Platelet count (109/L) | 324 | 150–450 |
| White cell count (109/L) | 16.3 | 2.2–10.0 |
| Neutrophil counts (109/L) | 12.8 | 1.0–5.0 |
| INR | 12.47 | 0.92–1.09 |
| APTT (s) | 89.5 | 26.4–38.1 |
| TT (s) | 16.64 | 12.8–17.6 |
| Fibrinogen (g/L) | 5.2 | 1.7–3.6 |
| CRP (mg/L) | 120 | 0–5 |
| ESR (mm/hour) | 114 | 0–20 |
| LDH (U/L) | 321 | 100–190 |
APTT, activated partial thromboplastin time; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; Hb, haemoglobin; INR, international normalised ratio; LDH, lactate dehydrogenase; MCH, mean corpuscular haemoglobin; MCV, mean corpuscular volume; TT, thrombin time.
ECHO revealed mild mitral regurgitation; otherwise, there was no vegetation or cardiac thrombus. CT angiography of the aorta and upper limbs revealed atherosclerotic changes of the aorta but there were no thrombi, aneurysms, collateralisation or signs of vasculitis. Moreover, duplex sonography of the upper extremity arteries was unremarkable.
The patient had an ultrasound-guided biopsy of the right cervical lymph node, and the histopathological examination showed the presence of a diffuse polymorphous infiltrate which was composed of small lymphoid cells, scattered eosinophils, plasma cells and histiocytes. There were scattered large cells, some of which showed the Reed-Sternberg cell morphology. In addition, some of the large cells were positive for CD3 and weakly positive for CD20 and PAX5. CD15 showed Golgi stain in rare cells and CD3-stained reactive T cells in the background with resetting around large cells. Epstein-Barr virus-encoded small RN was positive in large cells.
A positron emission tomography (PET) scan showed multiple fluorodeoxyglucose (FDG)-avid generalised lymphadenopathy with diffuse uptake in the spleen, the right adrenal gland and skeleton. There were multiple FDG-avid lung opacities with suspicious bilateral lung effusion (figure 2).
Figure 2.
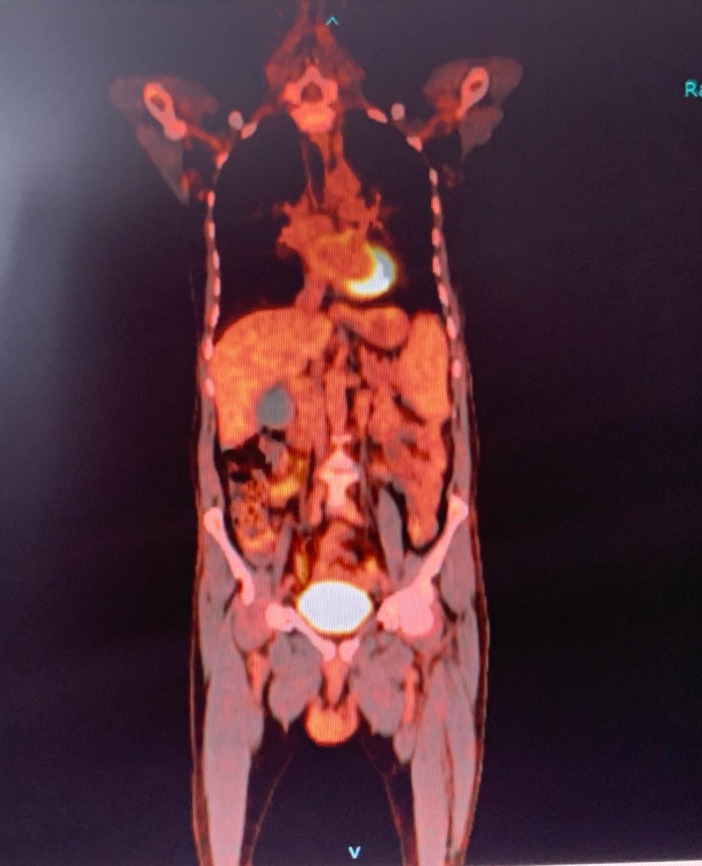
Positron emission tomography scan multiple fluorodeoxyglucose-avid generalised lymphadenopathy with diffuse uptake in the spleen and other organs.
Differential diagnosis
Given the history of fever, cardiac murmur and high inflammation markers, IE was suspected. However, the negative blood cultures and unremarkable ECHO findings ruled out this possibility. Furthermore, the presence of sinus rhythm and the absence of an intracardiac thrombus widely excluded an intracardiac thrombosis with thromboembolism phenomenon.
The patient was an active smoker, hence we considered thromboangiitis obliterans (Buerger’s disease), but the patient’s age and the absence of other features, including sensory involvement and typical radiological changes, ruled out this possibility.
Raynaud’s phenomenon presents as transient digital ischaemia caused by vasoconstriction, which can be exacerbated by exposure to stressful situations, vibrations or cold temperatures. The patient’s clinical presentation and rapid progression of digital ischaemia were inconsistent with Raynaud’s phenomenon.
Additional differential diagnoses were complex regional pain syndrome (CRPS) and reflex sympathetic dystrophic syndrome (RSDS), and both were unlikely causes given the absence of neurological complaints and deficits. Other causes of digital ischaemia are atherosclerosis, autoimmune conditions, connective tissue disease, vasculitis and hyperviscosity conditions such as cryoglobulinemia. However, the clinical presentation and complete work-up widely excluded all these differential diagnoses.
After reviewing the histopathological and radiological imaging reports, we concluded that the patient had stage IV Hodgkin’s lymphoma with a calculated International Prognostic Score of 4, which indicates a poor prognosis.
Treatment
The patient was given ceftriaxone and vancomycin due to the initial high index suspicion of IE. These medications were ceased after the negative microbiological work-up and the unremarkable ECHO findings. We ceased the warfarin and successfully corrected the coagulation profile using 2.5 mg of oral vitamin K given an absent clinical indication for therapeutic anticoagulation.
The vascular surgical team assessed the patient, and the acute ischaemic limb diagnosis was excluded. Besides quitting cigarette smoking, the patient was given aspirin (100 mg once daily), nifedipine (30 mg once daily), glyceryl dinitrate (40 mg SR once daily) and enoxaparin (40 mg daily) as a medical measure to treat the digital ischaemia (figure 1B).
On confirmation of the diagnosis and completion of the staging work-up, the patient was started on a first cycle of chemotherapy consisting of dacarbazine, doxorubicin and vinblastine.
Outcome and follow-up
Due to severe hyponatraemia and other side effects, the chemotherapy regimen was changed to bendamustine and dacarbazine. The patient received four cycles and the follow-up PET scan showed a significant, generalised reduction in the size and metabolic activities of the lymph nodes combined with a reduced uptake in the bone marrow, lungs, adrenal glands and spleen. Moreover, the patient had almost complete resolution of the digital ischaemia apart from dry gangrene of his right middle digit (figure 1C).
Discussion
Acral vascular syndrome is a spectrum of manifestations that could present as Raynaud’s phenomenon, acrocyanosis or acronecrosis.9 10 It is usually associated with connective tissue diseases, thromboembolic diseases, atherosclerotic diseases and hyperviscosity syndrome. It is rarely seen in malignancies.8 11
PAVS is a rare phenomenon associated with malignancy and characterised by rapid progression of digital ischaemia that can potentially lead to gangrene of the fingers.11 12 PAVS is more common in solid malignancies, especially adenocarcinoma of the breast and gastrointestinal system.5 9 11 13 14 The reported incidence of digital ischaemia in patients with cancer is two cases per 100 000 persons per year.2 15 Moreover, PAVS is more common in women who are older than 50 years of age,1 and may occur before, after or coincide with a cancer diagnosis.2 In previously reported cases of patients with PAVS, the prevalence of metastatic disease of the underlying malignancy was 64%.1
The pathophysiology of PAVS is not fully understood, and it is probably caused by multiple factors. It could be due to vaso-occlusion of the vessels caused by vasoconstrictive factors produced by the malignant cells.7 16 Furthermore, the compressive effect of the tumour on the cervical plexus might cause hyperstimulation of the sympathetic nervous system and vasospasm.3 Other proposed mechanisms are immune complex deposition, micro-fragments of the tumour cell embolisation, increased coagulability secondary to increased circulating procoagulant factors, spontaneous platelet aggregation or impaired fibrinolytic and anticoagulant pathways.5 8
Several medical and surgical therapies have been tried for the management of PAVS—including antiplatelets, anticoagulants, vasodilators, sympatholytics, pentoxifylline, nitroglycerin and surgical sympathectomy—resulting in variable outcomes.1 However, complete remission of digital ischaemia was reported in most cases with the treatment of the underlying malignancy.2 7 8
PAVS has been reported in very few cases of Hodgkin’s lymphoma.3–6 This case illustrates a very unusual presentation of Hodgkin’s lymphoma. In the clinical context of fever, cardiac murmur and high inflammatory markers, it was necessary to exclude IE. Our patient underwent multiple investigations as the differential diagnoses were broad. Thereafter, the possibilities of connective tissue diseases, vasculitis, thromboangiitis obliterans, atherosclerosis, CRPS, RSDS and cryoglobulinemia were excluded. The history of weight loss, chronic smoking, fever and extensive lymphadenopathy was important clues for the possibility of malignancy, which was confirmed later during the same admission.
Besides continuing with the diagnostic work-up and due to the rapid progression of the digital ischaemia, we initiated treatment targeting possible mechanisms of PAVS. This constituted of 100 mg of aspirin per day, 30 mg of nifedipine per day, 40 mg of glyceryl dinitrate slow-release tablet per day and 40 mg subcutaneous injection of enoxaparin per day. Along with the ongoing medical treatment, the patient successfully quit smoking, which probably helped to improve his digital ischaemia. All these measures resulted in some improvement in the extent of the digital ischaemia (figure 1B). However, the benefit cannot be uniformly expected with these treatments due to the complex pathophysiology underlying PAVS.1 Following the initiation of chemotherapy, the patient had further improvement in his digital ischaemia (figure 1C).
Learning points.
The presence of acral vascular syndrome—especially in older people—should alert the physician to search for an underlying malignancy.
The pathophysiology of paraneoplastic acral vascular syndrome is not fully understood and is likely caused by multiple factors.
Smoking cessation, vasodilators, antiplatelets and anticoagulants may improve the digital ischaemia in some cases, but outcomes vary.
Treatment of the underlying malignancy was associated with the resolution of vascular involvement in most of the cases.
Footnotes
Contributors: SA is the first author, she involved on writing up the manuscript and taking care of this patient during his admission. AHKA-B: worked on writing up and reviewing the manuscript. AMAA: worked on writing up and reviewing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chow SF, McKenna CH. Ovarian cancer and gangrene of the digits: case report and review of the literature. Mayo Clin Proc 1996;71:253–8. 10.4065/71.3.253 [DOI] [PubMed] [Google Scholar]
- 2.Le Besnerais M, Miranda S, Cailleux N, et al. . Digital ischemia associated with cancer: results from a cohort study. Medicine 2014;93:e47. 10.1097/MD.0000000000000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpern SM, Todd P, Kirby JD. Hodgkin's disease presenting with digital ischaemia. Clin Exp Dermatol 1994;19:330–1. 10.1111/j.1365-2230.1994.tb01207.x [DOI] [PubMed] [Google Scholar]
- 4.Solak Y, Aksoy S, Kilickap S, et al. . Acrocyanosis as a presenting symptom of Hodgkin lymphoma. Am J Hematol 2006;81:151–2. 10.1002/ajh.20479 [DOI] [PubMed] [Google Scholar]
- 5.Villano F, Peixoto A, Riva E, et al. . Digital ischemia as an unusual manifestation of Hodgkin's lymphoma. Case Rep Hematol 2018;2018:1980749. 10.1155/2018/1980749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poiraud C, Durant C, Saint-Jean M, et al. . [A rare cause of digital necrosis: Hodgkin's disease]. Presse Med 2011;40:432–5. 10.1016/j.lpm.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez Martín AM, Guirao Arrabal E, Jiménez Puya R, et al. . Paraneoplastic acral vascular syndrome. Actas Dermosifiliogr 2015;106:601–2. 10.1016/j.ad.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 8.Madabhavi I, Revannasiddaiah S, Rastogi M, et al. . Paraneoplastic Raynaud’s phenomenon manifesting before the diagnosis of lung cancer. Case Rep Child Meml Hosp Chic 2012;2012:2012 10.1136/bcr.03.2012.5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. . Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol 2002;47:47–52. 10.1067/mjd.2002.120474 [DOI] [PubMed] [Google Scholar]
- 10.Maharaj S, Chang S, Seegobin K, et al. . Paraneoplastic acral vascular syndrome. Cleve Clin J Med 2018;85:101–2. 10.3949/ccjm.85a.17007 [DOI] [PubMed] [Google Scholar]
- 11.Gambichler T, Strutzmann S, Tannapfel A, et al. . Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer 2017;17:327. 10.1186/s12885-017-3313-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdallah M, Hamzaoui S, Larbi T, et al. . [Etiological profile of digital necrosis of the upper limbs: analysis of 25 cases]. J Mal Vasc 2010;35:12–16. 10.1016/j.jmv.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Sahan C, Ucer T, Aksakal E. A case of hepatocellular carcinoma who admitted with Raynaud's phenomenon. Rheumatol Int 2006;27:87–9. 10.1007/s00296-006-0154-z [DOI] [PubMed] [Google Scholar]
- 14.Kopterides P, Tsavaris N, Tzioufas A, et al. . Digital gangrene and Raynaud's phenomenon as complications of lung adenocarcinoma. Lancet Oncol 2004;5:549. 10.1016/S1470-2045(04)01566-9 [DOI] [PubMed] [Google Scholar]
- 15.Carpentier PH, Guilmot JL, Hatron PY, et al. . [Digital ischemia, digital necrosis]. J Mal Vasc 2005;30:4S29–37. [PubMed] [Google Scholar]
- 16.Rodrigues T, Barcelos A. Digital ischemia as a paraneoplastic phenomenon. Acta Reumatol Port 2016;41:378–9. [PubMed] [Google Scholar]