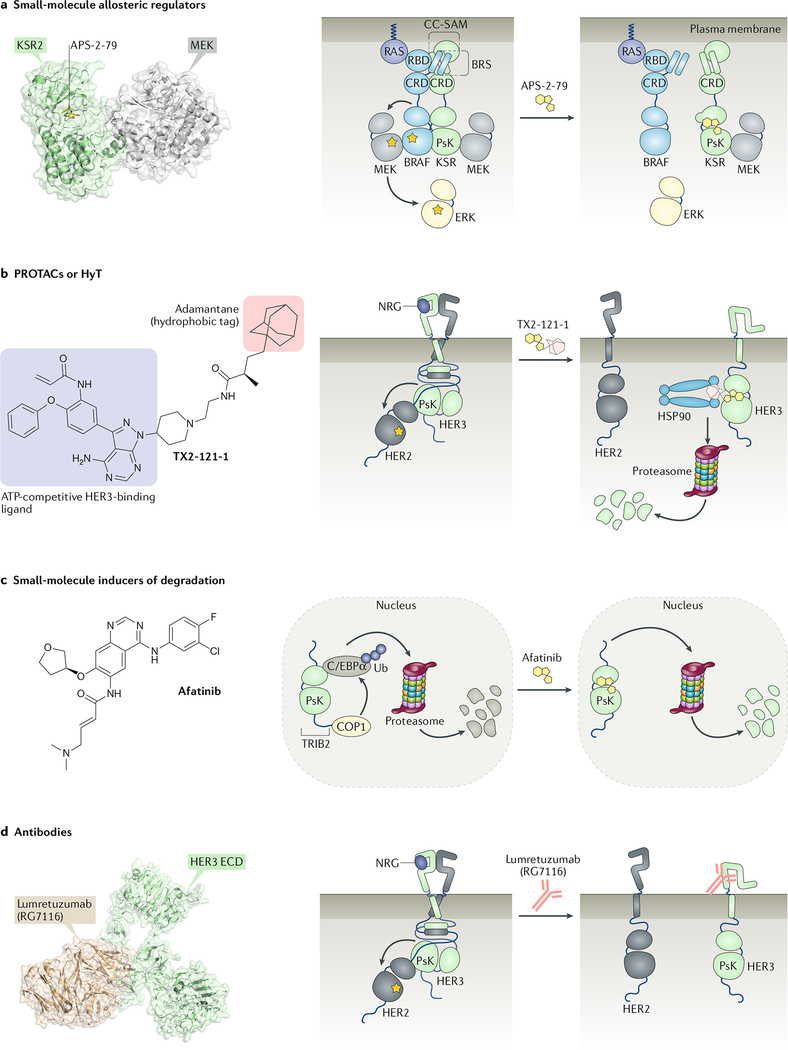
Fig. 5: Strategies for pharmacological targeting of pseudokinases.
a | Small molecules can be used to allosterically modulate the conformation and interactions of pseudokinases, as illustrated by APS-2–79, an ATP-competitive inhibitor of kinase suppressor of RAS 2 (KSR2), that stabilizes an inactive conformation of KSR2 that is incompatible with heterodimerization with RAF and that blocks RAF-dependent phosphorylation sites on MEK. A crystal structure of KSR2 bound to APS-2–79 and MEK is shown in the left panel. b | Proteolysis-targeting chimaeras (PROTACs) or hydrophobic tagging (HyT) can be used to induce degradation of a protein of interest. The human epidermal growth factor receptor 3 (HER3) inhibitor TX2–121-1 consists of a portion that binds covalently to the HER3 nucleotide-binding pocket (blue) connected to adamantane (red), a hydrophobic moiety that mimics the presence of an unfolded protein. Binding of TX2–121-1 leads to proteasomal degradation of HER3, a process that is aided by chaperones, including HSP70 and HSP90. c | Small molecules other than the bifunctional ligands employed for PROTAC-mediated or HyT-mediated protein degradation can be used to destabilize pseudokinases and induce their degradation. Covalent inhibitors of epidermal growth factor receptor (EGFR) and HER2, such as afatinib, destabilize the pseudokinase TRIB2 and promote its proteasomal degradation. d | Antibodies can be used to target the extracellular domains (ECDs) of receptor tyrosine kinases that possess pseudokinase domains. The monoclonal antibody lumretuzumab (RG7116) locks the HER3 ECD in an inactive conformation that prevents ligand binding. A crystal structure of an antigen-binding fragment, derived from lumretuzumab, bound to the HER3 ECD is shown in the left panel. C/EBPα, CCAAT enhancer-binding protein-α; CRD, cysteine-rich domain; NRG, neuregulin.