Abstract
The microbial fermentation process has been used as an alternative pathway to the production of value-added natural products. Of the microorganisms, Yarrowia lipolytica, as an oleaginous platform, is able to produce fatty acid-derived biofuels and biochemicals. Nowadays, there are growing progresses on the production of value-added fatty acid-based bioproducts in Y. lipolytica. However, there are fewer reviews performing the metabolic engineering strategies and summarizing the current production of fatty acid-based bioproducts in Y. lipolytica. To this end, we briefly provide the fatty acid metabolism, including fatty acid biosynthesis, transportation, and degradation. Then, we introduce the various metabolic engineering strategies for increasing bioproduct accumulation in Y. lipolytica. Further, the advanced progress in the production of fatty acid-based bioproducts by Y. lipolytica, including nutraceuticals, biofuels, and biochemicals, is summarized. This review will provide attractive thoughts for researchers working in the field of Y. lipolytica.
Keywords: Yarrowia lipolytica, cell factory, fatty acid metabolism, bioproducts, metabolic engineering
Introduction
With the growing crisis of oil energy, microbial production of biochemicals, as one potential alternative route, has received increasing attention (Levering et al., 2015; Ji and Huang, 2019; Ji and Ledesma-Amaro, 2020). Among these microorganisms, the oleaginous yeasts, such as Rhodosporidium toruloides, Lipomyces starkeyi, and Yarrowia lipolytica, are able to produce oleochemicals (Probst et al., 2016; McNeil and Stuart, 2018; Park et al., 2018b; Miller and Alper, 2019). Y. lipolytica, as Food and Drug Administration (FDA)-regarded Generally Recognized as Safe (GRAS) yeast with lipids over 20% of its biomass, performs many attractive characteristics and applications, including having mature genetic tools, secreting functional enzymes, and producing organic acids, lipids, and non-native chemicals (Xie, 2017; Darvishi et al., 2018; Larroude et al., 2018; Madzak, 2018; Ma et al., 2019). Currently, many researchers focus on the biotechnological application of Y. lipolytica (Xie et al., 2015; Markham et al., 2018; Robles-Rodriguez et al., 2018; Li et al., 2019). In particular, the different metabolic engineering strategies are applied in the lipid production for Y. lipolytica (Abdel-Mawgoud et al., 2018; Wang J. et al., 2020). In fact, Y. lipolytica is able to produce fatty acids in the form of lipids, either grown on hydrophilic or hydrophobic materials (Spagnuolo et al., 2018; Ma et al., 2020). Generally, these fatty acid-based bioproducts from Y. lipolytica are divided into three different types, based on the chain length, the terminal reductive state, and the modifications to the main chain of target product (Yan and Pfleger, 2020). With the development of metabolic engineering and synthetic biology, there are growing progresses on the production of value-added fatty acid-based bioproducts in Y. lipolytica. In the past 5 years, researchers have reviewed the production of fatty acid-derived products by Y. lipolytica, including fatty alkanes, fatty alcohols, and polyunsaturated fatty acids (PUFAs) (Ledesma-Amaro and Nicaud, 2016b; Ma et al., 2020). However, there is less review performing the metabolic engineering strategies for improving the production of fatty acid-based products and summarizing the current biosynthesis of fatty acid-based bioproducts in Y. lipolytica.
Herein, in this review, we describe a brief overview of the biochemistry metabolism of fatty acid in Y. lipolytica. Then, we focus on introducing the various metabolic strategies for increasing bioproduct accumulation, including constructing and engineering metabolic pathways, optimizing fermentation conditions, and engineering compartmentalization system. Moreover, we summarize the recent progress in the production of fatty acid-based bioproducts in Y. lipolytica, including nutraceuticals, biofuels, and biochemicals (Table 1). This article will provide attractive thoughts for researchers working in the field of Y. lipolytica.
TABLE 1.
Summary of the production of fatty acid-based bioproducts from the Y. lipolytica platform.
| Type | Target | Strain | Genetic manipulation | Production level | References |
| Nutraceuticals | DHA | Y. lipolytica Po1h:Af4 | Expression of artificial pfa-BGC version C1_V2. | 350 mg/L (after 300 h) | Gemperlein et al., 2019 |
| EPA | Y. lipolytica Y4305 | Expression of C16 elongase gene, Δ12- desaturase gene, Δ9- elongase gene, Δ8- desaturase gene, Δ5- desaturase gene, Δ17- desaturase gene. Deletion of PEX10 gene. | 56.6% of total fatty acid | Xue et al., 2013 | |
| EPA | Y. lipolytica Y4184 | Deletion of Ylsnf1. | 7.6% of the DCW | Seip et al., 2013 | |
| EPA | Y. lipolytica Z7344 | Expression of desaturases and elongases genes. Two-stage continuous fermentation. | 48% of total lipids | Xie et al., 2017 | |
| Trans-10, cis-12 CLA | Y. lipolytica Polh-1292oPAI-5 | Expression of PAI gene. | 5.9% of total fatty acid | Zhang B. X. et al., 2012 | |
| Trans-10, cis-12 CLA | Y. lipolytica Polh-1292-spopai-d12-16 | Expression of FADS12, d12 from Mortierella alpine and opai gene. | 16% of DCW | Zhang B. X. et al., 2013 | |
| CLA | Y. lipolytica JMY3479, CLIB 3039 | Overexpression of oPAI and Δ12-desaturase from Mortierella alpine | 302 mg/L | Imatoukene et al., 2017 | |
| Trans-10, cis-12 CLA | Y. lipolytica WXYL037 | Overexpression of inherent diacylglycerol transferase gene, Δ12-desaturase from Mortierella alpina and isomerase gene from Propionibacterium acnes. | 132.6 mg/L | Wang et al., 2019 | |
| GLA | Y. lipolytica pYLd6d12 | Co-expression of fungal Δ6-desaturase and Δ12-desaturase genes | 20% of GLA from endogenous LA and OA | Chuang et al., 2010 | |
| GLA | Y. lipolytica Po1f-6-D | Expression of Δ6-desaturase gene from Mortierella alpine | 71.6 mg/L | Sun et al., 2017 | |
| ARA | Y. lipolytica YL 6-1 | Expression of Δ6-desaturase, Δ6-elongase and Δ5- desaturase from Mortierella alpine. | 0.4% of total lipids | Liu et al., 2017a | |
| ARA | Y. lipolytica YL 6-1 | Transfer extracellular organic acids to the synthesis of intracellular ARA. | 0.42% of total lipids | Liu et al., 2017b | |
| ARA | Y. lipolytica RH-4 | Enzyme fusion of Δ9- elongase and Δ8- desaturase with the rigid linker (GGGGS) | 118.1 mg/L | Liu H. H. et al., 2019 | |
| RA | Y. lipolytica JMY2556 | Expression of CpFAH12 from C. purpurea. Overexpressing the native LRO1. | 43% of total lipids | Beopoulos et al., 2014 | |
| RA | Y. lipolytica CYLxR | Overexpression of SCD1, DGA1, LIP2 and CpFAH12. | 2.2 g/L | Guo et al., 2018 | |
| Odd-chain FAs (C17:1) | Y. lipolytica CCY 29-26-36 | Utilization propionate as substrate. | 38% of total lipids | Kolouchová et al., 2015 | |
| Odd-chain FAs (mainly C15:0, C17:0 and C17:1) | Y. lipolytica JMY3776 | Overexpression of ADH5. Deletion of ADH6 | 0.57 g/L, 0.75 g/L (Fed-batch) | Park et al., 2018a | |
| Odd-chain FAs | Y. lipolytica JMY7412 | Overexpression of the aspartate/α-ketobutyrate pathway | 0.36 g/L | Park et al., 2020 | |
| Biofuels | Fatty alcohols (C10) | Y. lipolytica Δpex10:FATcpa/FAR | Overexpression of FAR from Arabidopsis thaliana and FAT from C. palustris. Deletion of the major peroxisome assembly factor Pex10. | Over 500 mg/L | Rutter and Rao, 2016 |
| Fatty alcohols (C16) | Y. lipolytica Tafar1-5copy-Δdga1 fao1 strain | Expression of FAR gene from Barn owl. | 636.89 mg/L (intracellular), 53.32 mg/L (extracellular) | Wang et al., 2016 | |
| Fatty alcohols | Y. lipolytica Maqu2220-EcfadD | Expression of fatty acyl-CoA reductase Maqu2220 from Marinobacter aquaeolei and fadD from E. coli. Compartmentalization | 2.15 g/L (in a 3-L bioreactor) | Xu et al., 2016 | |
| FAEE | Y. lipolytica AD strain | Expression of Acinetobacter baylyi ADP1 wax-ester synthase AbAtfA. Overexpression of a peroxisomal/mitochondrial carnitine acyltransferase, perCat2. Mixtures of dextrose and canola oil. Compartmentalization | 142.5 mg/L | Xu et al., 2016 | |
| FAEE | Y. lipolytica GQY20 | Expression of WS gene from Marinobacter sp. Deletion of PEX10 gene. | 1.18 g/L (containing 5 vol% ethanol) | Gao et al., 2018 | |
| FAEE | Y. lipolytica YL6 | Expression of pdc and adhB from Z. mobilis and maqu_0168 from Marinobacter sp. Deletion of mfe1, gut2, pex10. With vegetable cooking oils (VCOs). | 82 mg/L | Ng et al., 2019 | |
| FAEE | Y. lipolytica Po1g:pYLP1A1GAMh and S288C | Expression of PDC1, ADH1, GAPDH and MhAtfA. Co-culture. | 4.8 mg/L | Yu et al., 2020 | |
| C19 cyclopropanated fatty acids | Y. lipolytica ENGR-HPH:ycoCFA-NAT:ycoCFA | Expression of CFA synthase from E. coli. | 3.03 g/L | Markham and Alper, 2018 | |
| FFAs | Y. lipolytica JMY5743 | Overexpression of DGA2, TGL4, KlTGL3. Deletion of faa1, mfe1. | 10.4 g/L | Ledesma-Amaro et al., 2016 | |
| FFAs | Y. lipolytica AD strain | Overexpression of hybrid hFAS-EcTesA. | 9.67 g/L (in a 3-L bioreactor) | Xu et al., 2016 | |
| FFAs | Y. lipolytica Y-4311 | Overexpression of ACC1. Deletion of gpd1, gut2, pex10. | 2033.8 mg/L | Yuzbasheva et al., 2018 | |
| Alkanes (C5) | Y. lipolytica PO1f-Δmfe1 | Deletion of mfe1. | 4.98 mg/L | Blazeck et al., 2013 | |
| Alka(e)nes | Y. lipolytica AD strain | Expression of MmCAR, BsuSfp and PmADO. | 23.3 mg/L | Xu et al., 2016 | |
| Alkenes (mainly C15 and C17) | Y. lipolytica S07004 | Expression of CvFAP (S121F) from Chlorella variabilis. Utilization half-light intensity. | 58.7 mg/L (Fed-batch) | Bruder et al., 2019 | |
| Biochemicals | γ-decalactone | Y. lipolytica PO1d strain | Expression of acyl-CoA oxidase gene. | 16.3 mg/g⋅h | Pagot et al., 1997 |
| γ-decalactone | Y. lipolytica Δpox2Δpox3 | Deletion of POX1 and POX5 genes. | 170 mg/L (2 L bioreactor) | Wache et al., 2001 | |
| γ-decalactone | Y. lipolytica JMY185 | Possession of multiple copies of POX2 gene. Deletion of POX3 and POX5 genes. | 150 mg/L | Waché et al., 2002 | |
| γ-decalactone | Y. lipolytica W29 | Increase O2 solubility | 300 mg/L (2 L bioreactor) | Aguedo et al., 2005 | |
| γ-decalactone | Y. lipolytica W29 | Oxygen mass transfer in a biphasic medium. | 141 mg/L (2 L bioreactor) | Gomes et al., 2007 | |
| γ-decalactone | Y. lipolytica W29 | Optimization operating conditions of substrate concentration, biotransformation start-up procedure and oxygen transfer. | 87 mg/g⋅h | Gomes et al., 2010 | |
| γ-decalactone | Y. lipolytica W29 | Strategies of fed-batch culture. | 73 mg/g (Intermittent fed-batch) | Gomes et al., 2012 | |
| γ-decalactone | Y. lipolytica ATCC20460 | Cell Immobilization. | 1597 mg/L | Braga and Belo, 2013 | |
| γ-decalactone | Y. lipolytica DSM 3286 | Supply of oxygen | 220 mg/L (Fed-batch) | Moradi et al., 2013 | |
| γ-decalactone | Y. lipolytica G3-2.21 | Genome shuffling of the haploid cells and the parent strains CGMCC 2.1405. | 3.75 g/L | Zhao et al., 2014 | |
| γ-decalactone | Y. lipolytica W29 | The direct influence of oxygen transfer rate. | 215 g/L (Fed-batch) | Braga and Belo, 2014 | |
| γ-decalactone | Y. lipolytica w-YLG | Cell immobilization in attapulgite along with the use of ionic liquid as a cosolvent. | 8.05 g/L (Fed-batch) | Zhao et al., 2015 | |
| γ-decalactone | Y. lipolytica CCMA 0242 | Optimization of cultivation conditions. | 0.128 g/L | Pereira de Andrade et al., 2017 | |
| γ-decalactone | Y. lipolytica CCMA 0357 | Optimization of cultivation conditions. | 3.5 g/L | Soares et al., 2017 | |
| γ-decalactone | Y. lipolytica CGMCC 2.2087 | Cell immobilization with BC-ALG carriers. | 8.37 g/L | Zhang et al., 2020 | |
| δ-decalactone | Y. lipolytica KCTC 17170 | Expression of linoleate 13-hydratase from L. acidophilus. | 16.3 mg/(L⋅h) | Kang et al., 2016 | |
| HFAs (ω-HDDA) | Y. lipolytica H222ΔPΔAΔF | Deletion of POX1-6, all relevant ADH genes and FAO1. | 7.9 g/L | Gatter et al., 2014 | |
| DCAs (C12) | H222ΔP | Deletion of POX1-6. | 11 g/L | Gatter et al., 2014 | |
| DCAs (C12) | Y. lipolytica iYLI647 | In silico model-based metabolic engineering. | ND | Mishra et al., 2018 | |
| DCAs (C12) | Y. lipolytica MTLY 37 | Deletion of pox2, pox3, pox4, pox5. | 20 mg/mL | Smit et al., 2005 | |
| Hexanal | Y. lipolytica PO1d-HPL | Expression of HPL gene. | 350 mg/L (Reaction medium) | Bourel et al., 2004 | |
| Hexanal | Y. lipolytica JMY 861 | Expression the hydroperoxide lyase (HPL) gene from green bell pepper fruit. Under oxido-reducing conditions. | 600 mg/L | Santiago-Gómez et al., 2009 | |
| Hexanal | Y. lipolytica JMY 861 | Overexpression of ADH from S. cerevisiae. | Increased by 84.1% | Aziz et al., 2016 | |
| CFA (C17 and C19) | Y. lipolytica JMY 6068 | Expression of CFAs from E. coli. | 2319 mg/L | Czerwiec et al., 2019 |
ND, not determine.
Biochemistry of Fatty Acid Metabolism
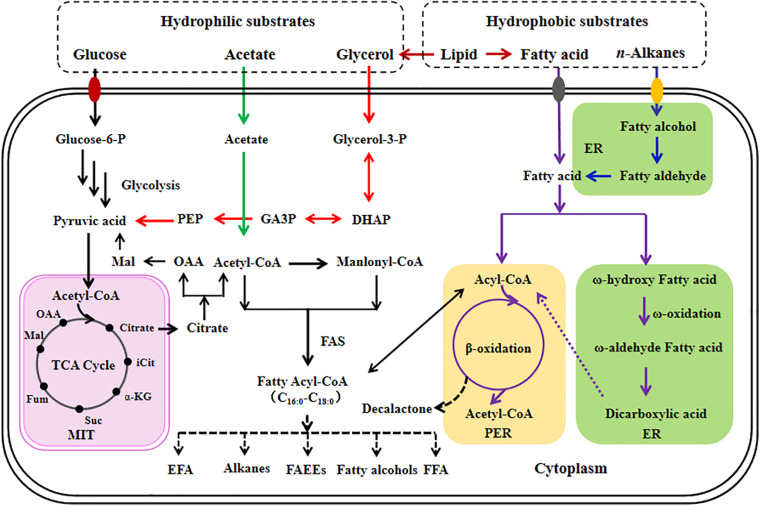
Currently, some articles have summarized the fatty acid metabolism of Y. lipolytica (Fickers et al., 2005; Abghari and Chen, 2014; Ledesma-Amaro and Nicaud, 2016a; Lazar et al., 2018). Previously, we reviewed in detail the characteristics of Y. lipolytica grown on various carbon substrates (Liu et al., 2015). Herein, the metabolism of fatty acid for producing its derived chemicals in Y. lipolytica is shown in Figure 1.
FIGURE 1.
Overview of fatty acid metabolism for the production of its based chemicals in Y. lipolytica. Different colored arrows are used to represent different metabolic pathways; black, de novo fatty acid metabolic pathway; green, acetate metabolic pathway; red, glycerol metabolic pathway; dark red, heterologous lipid metabolic pathway; purple, ex novo fatty acid metabolic pathway; blue, heterologous alkane metabolic pathway. Pathway localization with respect to specific subcellular organelles are also depicted. ER, endoplasmic reticulum; PER, peroxisome; MIT, mitochondria; TCA cycle, tricarboxylic acid cycle; DHAP, dihydroxyacetone phosphate; GA3P, glycerol-3-phosphate; iCit, isocitrate; α-KG, α-ketoglutarate; Suc, succinate; Fum, fumarate; Mal, malate; OAA, oxaloacetate; FAS, fatty acid synthase; EFA, essential fatty acid; FFA, free fatty acid; FAEEs, fatty acid ethyl esters.
Fatty Acid Biosynthesis
With the development of metabolic engineering, it enables Y. lipolytica to utilize a wide range of carbon sources (Liu et al., 2015; Ledesma-Amaro and Nicaud, 2016b). Using hydrophilic substrates (such as glucose and glycerol) as carbon source, fatty acid is synthesized by de novo pathway in Y. lipolytica. With glucose as sole carbon source, it is converted into pyruvate via the glycolytic pathway in the cytosol. Then, pyruvate is transported to mitochondria and transformed into acetyl-CoA. Acetyl-CoA, a key precursor involved in fatty acid biosynthesis, can be produced by different metabolic routes, including citrate degradation catalyzed by ATP citrate lyase (ACL), fatty acid degradation from β-oxidation pathway, acetate transformation by acetyl-CoA synthetase (ACS, YALI0F05962p), and pyruvate transformation by pyruvate dehydrogenase complex. Under nitrogen-limited conditions, citrate is secreted into cytosol from mitochondria in Y. lipolytica and acetyl-CoA is produced by ACL catalysis. In Y. lipolytica, ACL is encoded by ACL1 (YALI0E34793p) and ACL2 (YALI0D24431p). Further, acetyl-CoA is transformed into malonyl-CoA by acetyl-CoA carboxylase (ACC, YALI0C11407p). Generally, acetyl-CoA and malonyl-CoA are used as substrates for fatty acid biosynthesis by fatty acid synthetases (FAS, YALI0B15059p, and YALI0B19382p) in Y. lipolytica. Naturally, Y. lipolytica can only produce C16 and C18 fatty acids (Beopoulos et al., 2009). Notably, the inherent long-chain PUFAs, including oleic acid (OA, C18:1) or linoleic acid (LA, C18:2), are synthesized by desaturase located in endoplasmic reticulum (ER).
Using hydrophobic materials (such as fats) as substrate, fatty acids are synthesized by ex novo pathway in Y. lipolytica. Generally, the extracellular fatty acids from the metabolism of hydrophobic materials are directly transported to cytosol in Y. lipolytica. Then, fatty acids are converted into derived chemicals by the corresponding oxidation process. Additionally, using alkane from oil refinery as carbon source, fatty acids are synthesized by the enzyme catalytic system located in ER, including cytochrome P450 reductase (EC 1.6.2.4), fatty alcohol oxidase (EC 1.1.3.20), and fatty aldehyde dehydrogenase (EC 1.2.1.3).
NADPH is an important reducing power involved in fatty acid biosynthesis in Y. lipolytica. Generally, there are two identified routes for providing NADPH pool in Y. lipolytica (Qiao et al., 2017). One route is from decarboxylation reaction catalyzed by malic enzyme (EC 1.1.1.40) that occurred in cytosol; the other metabolic route is from the pentose phosphate pathway in Y. lipolytica. Previously, it was reported that overexpression of malic enzyme has little impact on lipid accumulation in Y. lipolytica (Beopoulos et al., 2011; Zhang H. et al., 2013). Wasylenko et al. (2015) reported that the oxidative pentose phosphate pathway, harboring glucose 6-phosphate dehydrogenase (EC 1.1.1.49) and 6-phosphogluconolactonase (EC 3.1.1.31), is the primary source of lipogenic NADPH in Y. lipolytica.
Fatty Acid Transportation
To date, the mechanism of fatty acid transportation is unclear in Y. lipolytica. Generally, shorter carbon-chain fatty acids, such as C8:0 and C10:0, are toxic for Y. lipolytica. Using primrose oil containing C18 fatty acids as substrate, Y. lipolytica performs a higher assimilation rate for unsaturated fatty acids (C18:3, C18:2, and C18:1) than that for saturated fatty acid (C18:0) (Aggelis et al., 1997). In this research, it was deduced that the fatty acids with different saturated levels are assimilated and transported via a selective uptake mechanism in Y. lipolytica. Recently, Dulermo et al. (2015) proposed a model of fatty acid transportation with chain length preferences in Y. lipolytica. According to this model, the extracellular fatty acids are transported into Y. lipolytica via unidentified transporters. Then, the internal fatty acids are activated to acyl-CoA by YlFaa1p (YALI0D17864p) or transported into peroxisome by unknown transporters. Notably, the activated fatty acids can be sorted in the form of triacylglycerols or enter peroxisome via transporters YlPxa1p (YALI0A06655p) and YlPxa2p (YALI0D04246p). Importantly, fatty acids from lipid remobilization can enter the peroxisome via transporter YlFat1p (YALI0E16016p).
In particular, the intracellular medium-chain fatty acids (C12–C14) are converted into fatty acyl-CoAs by fatty acyl-CoA synthetase II in the peroxisome for further degradation, whereas long-chain fatty acids (C16–C18) are converted into fatty acyl-CoA by fatty acyl-CoA synthetase I in the cytosol (Dulermo et al., 2015). Then, long-chain fatty acyl-CoA is either transported into peroxisome from cytosol or used as substrate for triacylglyceride biosynthesis in Y. lipolytica.
Fatty Acid Degradation
Generally, fatty acids, either from intracellular triacylglyceride hydrolysis or from extracellular fatty acid transportation, can be transformed into fatty acid-based chemicals by oxidation in Y. lipolytica. Notably, the intracellular fatty acids are mainly degraded by peroxisomal β-oxidation or ω-oxidation pathway. In fact, the intracellular fatty acids from lipid remobilization are mainly converted into acetyl-CoA, via peroxisomal β-oxidation pathway. In particular, each cycle of β-oxidation consists of a four-step enzyme catalyzed reaction in Y. lipolytica. The first step is catalyzed by acyl-CoA oxidases (EC 1.3.3.6), the second step and third steps are catalyzed by multifunctional enzyme, and the last step is catalyzed by 3-ketoacyl-CoA thiolase (EC 2.3.1.16). In addition, the intracellular fatty acids can be degraded into derived chemicals by ω-oxidation pathway that occurred in ER. The fatty acids are firstly converted into ω-hydroxyl-fatty acids by cytochrome P450-containing fatty acid ω-hydroxylase. Then, ω-hydroxyl-fatty acids are converted into ω-aldo-fatty acids by fatty alcohol dehydrogenase or fatty alcohol oxidase, and ω-aldo-fatty acids are converted into long-chain diacids by fatty aldehyde dehydrogenase. In particular, the β-oxidation pathway can be engineered to synthesize β-hydroxy fatty acid (HFA) and lactones, whereas the ω-oxidation pathway can be engineered to produce ω-HFA and α, ω-dicarboxylic acids (DCAs) in Y. lipolytica.
Engineering Strategies to Increase Oleochemical Production
Nowadays, different metabolic strategies have been used to de novo produce the novel fatty acid-based bioproducts and accumulate the production of these derived biochemicals in Y. lipolytica (Table 2).
TABLE 2.
Engineering strategies to improve fatty acid-based bioproducts accumulation in Y. lipolytica.
| Engineering strategies | Bioproducts | Strategy details | References |
| Constructing and engineering metabolic pathways | EPA | Constructing synthetic pathways | Xue et al., 2013 |
| Lipids | Improving acetyl-CoA supplement | Xu et al., 2016 | |
| Lipids | Increasing NADPH availability | Qiao et al., 2017 | |
| Trans-10, cis-12 CLA | Overexpressing the endogenous enzymes | Wang et al., 2019 | |
| Fatty alcohols | Eliminating downstream degradation | Rutter and Rao, 2016 | |
| Optimizing fermentation conditions | γ-decalactone | Improving oxygen transfer | Moradi et al., 2013 |
| GLA | A temperature-shift strategy of cultivation | Sun et al., 2017 | |
| CLA | Changing the medium components | Wang et al., 2019 | |
| EPA | Two-stage continuous fermentation | Xie et al., 2017 | |
| Engineering compartmentalization system | FAEE | Endoplasmic reticulum or peroxisome localization | Xu et al., 2016 |
| Alkane | Endoplasmic reticulum or peroxisome localization | ||
| Fatty alcohol | Peroxisome localization | ||
| γ-decalactone | Cell immobilization | Zhang et al., 2020 |
Constructing and Engineering Metabolic Pathways
Researchers have focused on constructing and optimizing metabolic pathways to achieve efficient fatty acid and its derivatives biosynthesis in Y. lipolytica, using various metabolic engineering strategies, including constructing heterologous synthetic pathways, overexpressing endogenous enzymes. Naturally, Y. lipolytica can produce linoleic acid as the precursor of ω-3/6 fatty acids (Liu et al., 2017a). Generally, the novel linoleic acid-derived nutraceuticals, such as arachidonic acid (ARA, C20:4) and eicosapentaenoic acid (EPA, C20:5), can be de novo synthesized via constructing the synthetic pathway in Y. lipolytica. For example, to de novo produce EPA in Y. lipolytica, the selected and optimized multiple copies of different chimeric genes from different microorganisms were integrated into yeast genome (Δ9-elongase, Δ8-desaturase, and Δ5-desaturase from E. gracilis, C16/18-elongase from M. alpina, Δ12-desaturase gene from F. moniliforme, Δ17-desaturase from P. aphanidermatum, and CPT), which led to the first engineered commercial strain Y4305 under strong promoters, containing 30 copies of nine different genes, which can produce EPA at 56.6% of the total fatty acids (TFA), without γ-linolenic acid (GLA, C18:3) accumulation (Xue et al., 2013).
Through overexpressing and eliminating the endogenous enzymes involved in the lipid degradation, the accumulation of fatty acid and its derivatives has been greatly enhanced in Y. lipolytica (Dulermo and Nicaud, 2011). Generally, the availability of precursors, including acetyl-CoA and NADPH, limits the lipid biosynthesis. Previously, by harnessing the carnitine shuttle mechanism, the lipid titer was enhanced 1.75-fold via increasing acetyl-CoA supplement (Xu et al., 2016). Qiao et al. (2017) performed a specific strategy of converting NADH to NADPH in 13 engineered strains of Y. lipolytica for improving lipid synthesis. Recently, Wang et al. (2019) showed that the increased conjugated linoleic acid (CLA, C18:2) accumulation is reached by overexpressing the endogenous diacylglycerol transferase gene. Additionally, in order to block the lipid degradation in Y. lipolytica, Rutter and Rao (2016) showed that the peroxisome assembly factor Pex10 is the major enzyme involved in the peroxisomal β-oxidation or ω-oxidation pathway.
Optimizing Fermentation Conditions
The optimization of fermentation process, based on the microbial physiology, plays a key role in achieving the high titer, yield, and productivity of value-added products. Naturally, pH, temperature, and medium components are the common optimized approaches during the fermentation process of Y. lipolytica. Previously, the temperature-shift strategy of cultivation was successfully exhibited to increase GLA accumulation in Y. lipolytica (Sun et al., 2017). Recently, the production of CLA was increased by changing carbon and nitrogen source, carbon-t- nitrogen mass ratio, and CaCl2 concentrations (Wang et al., 2019). In addition, the fed-batch fermentation approach has been used to increase the production of drop-in biochemicals (Park et al., 2018a; Bruder et al., 2019). Compared with the continuous fermentation processes, the batch and fed-batch processes perform lower volumetric productivities (Li et al., 2011). In fact, the productivities utilizing continuous fermentation processes were improved, typically at the cost of product concentration, conversion yield, or both (Ethier et al., 2011). Previously, the novel two-stage continuous process for EPA accumulation in Y. lipolytica was developed (Xie et al., 2017). In this research, compared with the single-stage continuous and fed-batch fermentation, the novel continuous process, equipped with a small growth tank (Stage 1) and a large production tank (Stage 2), successfully improved the volumetric lipid productivities by 80%.
Generally, Y. lipolytica requires a high oxygen supply in the large-scale bioprocess. Previously, researchers have showed that the heterologous expression of gene encoding the bacterial hemoglobin from Vitreoscilla stercoraria (VHb) can improve the oxygen utilization efficiency and further increase the productivity (Suen et al., 2014; Zhang et al., 2017). Recently, Mirończuk et al. (2019) performed that the improved erythritol synthesis is obtained in Y. lipolytica, by overexpressing the codon-optimized bacterial hemoglobin (VHb). Through improving oxygen transfer rate using higher agitation rates or pure oxygen for aeration, the production of γ-decalactone was successfully enhanced (Moradi et al., 2013).
Engineering Compartmentalization System
Naturally, each subcellular compartment in Y. lipolytica provides a unique microenvironment, including enzyme, precursor, and cofactor composition. Due to the distinct organelle characteristics, the separation of organelles in the cytosol performs the potential to eliminate metabolic crosstalk and enhance compartmentalized pathway efficiency (Hammer and Avalos, 2017). Previously, Xu et al. (2016) reported that the titer of drop-in product performs a 10–15-fold improvement, by targeting the fatty acid ethyl ester (FAEE) pathway to either ER or peroxisome of Y. lipolytica. Compared to free cell systems, the immobilized cells could tolerate unsuitable conditions (Li et al., 2009; Macario et al., 2009). For example, using cell immobilization systems with bacterial cellulose-alginate (BC-ALG) carriers, γ-decalactone production was successfully reached with 8.37 g/L in the repeated experiments in Y. lipolytica, an approximately 3.7-fold improvement over with an ALG carrier alone (Zhang et al., 2020).
Modular co-culture metabolic engineering combines the strains carrying each pathway module in the engineered strains to form a synthetic complex, which can accommodate different modules expressing functional genes in different hosts to produce drop-in bioproducts (Jawed et al., 2019). Recently, by coculturing and engineered Y. lipolytica and S. cerevisiae strain, a synthetic microbial consortium was constructed to increase the titer of FAEE. In this research, the titer of FAEE biodiesel at 4.8 mg/L was reached by the synthetic microbial consortium under the optimum coculture conditions (Yu et al., 2020).
Production of Fatty Acid-Based Bioproducts
Nutraceuticals
Due to the potential applications of microbial lipids in the field of food supplements, the microbial production of PUFAs is becoming an industrial reality (Bellou et al., 2016). Of these oleaginous yeasts, Y. lipolytica can synthesize OA and LA.
Omega-3 PUFAs with special function, particularly α-linolenic acid (ALA, C18:3), EPA, and docosahexaenoic acid (DHA, C22:6), are gaining importance. Previously, using inherent LA as carbon substrate, Xue et al. (2013) constructed an engineered Y. lipolytica strain Y4305 capable of de novo producing EPA at 56.6% of TFA, by the combined metabolic engineering strategies. With Y. lipolytica as a host, the highest titer of ALA at 1.4 g/L was produced in the engineered strain containing a bifunctional Δ12–Δ15 desaturase from Rhodosporidium kratochvilovae, under the optimized fermentation conditions (Cordova and Alper, 2018). Recently, an artificial PUFA biosynthetic gene clusters, encoding DPA/DHA-type PUFA synthases, was expressed in Y. lipolytica. In this research, under the optimized fermentation process, the DHA level over 350 mg/L was reached (Gemperlein et al., 2019).
Omega-6 PUFAs, including conjugated CLA, GLA, and ARA, are a major family of PUFAs with diverse bioactivities (Xu and Qian, 2014). In 2017, the combined elimination of β-oxidation pathway and overexpression of Δ12-desaturase was conducted in Y. lipolytica, which leads to CLA production at 302 mg/L (Imatoukene et al., 2017). Recently, Wang et al. (2019) showed that the maximum content of trans-10, cis-12 CLA at 132.6 mg/L is reached by the engineered Y. lipolytica under the optimized fermentation conditions, by the overexpression of inherent diacylglycerol transferase from Y. lipolytica, Δ12 desaturase from Mortierella alpina, and Propionibacterium acnes isomerase. With LA as substrate, the GLA biosynthetic pathway was constructed in Y. lipolytica harboring Δ6-desaturase from M. alpina. Under the optimized fermentation process, the titer of GLA at 71.6 mg/L was achieved (Sun et al., 2017).
Arachidonic acid (ARA, C20:4) is also an essential ω-6 PUFA with special functions. Previously, we developed the in vivo one-step pathway assembly and integration method enabling Y. lipolytica to produce ARA (Liu et al., 2017a). Additionally, we showed that the ARA biosynthetic pathway is able to redirect the carbon flux toward intracellular fatty acid accumulation at the expense of extracellular organic acid secretion in the engineered Y. lipolytica strain (Liu et al., 2017b). Recently, using Δ9 elongase pathway engineering and fusion enzyme strategy, the ARA titer at 118.1 mg/L was achieved in the engineered Y. lipolytica (Liu H. H. et al., 2019).
Ricinoleic acid (RA, C18:1) and its derivatives perform oleochemical applications, due to the special characteristics. Meesapyodsuk and Qiu (2008) first identified an oleic acid-like hydroxylase (CpFAH12) from Claviceps purpurea. Previously, with LA as substrate, an engineered Schizosaccharomyces pombe strain capable of producing RA, harboring heterologous CpFAH12 from C. purpurea, was constructed (Holic et al., 2012). Using Y. lipolytica as a host, Beopoulos et al. (2014) reported that RA accumulation at 42% of total lipids is achieved, by overexpressing C. purpurea Δ12-hydroxylase and native Y. lipolytica Lro1p acyltransferase. Recently, by the combined overexpression of SCD1 gene encoding stearoyl-CoA desaturase, DGA1 gene encoding acyl-CoA:diacylglycerol acyltransferase, LIP2 gene encoding lipase, and CpFAH12 gene encoding hydroxylase, the production level of RA at 2.2 g/L was obtained by the engineered Y. lipolytica using cellulose as substrate (Gao et al., 2018).
Odd-chain fatty acids with special biochemical and biological activities are receiving growing attention on potential applications (Řezanka and Sigler, 2009). Previously, Kolouchová et al. (2015) performed that Y. lipolytica is capable of producing heptadecenoic acid (C17:1) using propionate as substrate. Recently, the deletion of the PHD1 gene and optimization of the fermentation process were applied to produce odd-chain fatty acids (mainly C15:0, C17:0, and C17:1) by Y. lipolytica grown on propionate (Park et al., 2018a). Additionally, Park et al. (2020) constructed an engineered Y. lipolytica capable of de novo producing odd-chain fatty acids, using glucose as sole substrate without any propionate supplementation.
Biofuels
The microbial production of fatty alcohols is becoming an alternative method to meet the increasing demand. Presently, various microorganisms, such as Escherichia coli and Saccharomyces cerevisiae, have been engineered for fatty alcohol production (Zhang et al., 2011; Zhou et al., 2016). Using Y. lipolytica as a host, Wang et al. (2016) constructed a novel fatty alcohol-producing workhorse, harboring Tafar1 gene coding fatty acyl-CoA reductase. Under the optimized tri-module condition, the intracellular hexadecanol at 636.89 mg/L and extracellular hexadecanol at 53.32 mg/L was produced, respectively. Meanwhile, through the overexpression of fatty acyl-ACP-thioesterases and fatty acyl-CoA reductase, and deletion of the major peroxisome assembly factor Pex10, the medium-chain alcohol, especially 1-decanol over 500 mg/L, was produced in the engineered Y. lipolytica (Rutter and Rao, 2016).
Researchers have performed that FAEEs or fatty acid methyl esters (FAMEs) can be produced via the microbial fermentation, using E. coli and S. cerevisiae (Steen et al., 2010; Nawabi et al., 2011; Yu et al., 2012). Fortunately, Xu et al. (2016) reported that the highest titer of FAEEs at 142.5 mg/L is produced in the engineered Y. lipolytica, using the compartmentalized metabolic engineering. Recently, an engineered Y. lipolytica strain, harboring the heterogenous pyruvate decarboxylase (pdc), alcohol dehydrogenase II (adhB) from Zymomonas mobilis, and wax ester synthases from Marinobacter sp., was constructed for producing FAEE. In this research, the titer of FAEE up to 82 mg/L was achieved by the supplementation of vegetable cooking oil (Ng et al., 2019). Meanwhile, Yu et al. (2020) developed the synthetic co-culture system comprising the engineered S. cerevisiae and Y. lipolytica strain, which was able to produce FAEE at 4.8 mg/L. To overcome the limitation of oxidative stability in the traditional FAMEs, Markham and Alper (2018) first performed the production of C19 cyclopropanated fatty acids in the engineered Y. lipolytica strain, harboring the heterologous cyclopropane fatty acid synthase from E. coli. In this research, the titer of C19 cyclopropanated fatty acids over 3.0 mg/L was produced under the bioreactor fermentation.
Free fatty acids (FFAs) are special oleochemicals with wide applications in the field of agricultural chemicals, soaps, and surfactants. Previously, Zhou et al. (2016) engineered S. cerevisiae capable of producing FFAs. Using Y. lipolytica as a workhorse, FFAs up to 9.67 g/L were produced by the engineered strain under the bioreactor scale with pH control (Xu et al., 2016). With the mixture of glucose and glycerol as carbon source, Yuzbasheva et al. (2018) showed that the engineered Y. lipolytica Y-4311 strain can produce FFAs (2033.8 mg/L) by the addition of dodecane.
Alka(e)nes are the major components of gasoline, diesel, and jet fuel. Presently, many studies have explored that the microbial production of alkanes is a conceivable method (Choi and Lee, 2013; Zhou et al., 2016). Using Y. lipolytica as a host expressing soybean lipoxygenase enzyme, Blazeck et al. (2013) first developed a microbial platform capable of producing pentane. In particular, in this research, using LA as substrate, the high titer of pentane at 4.98 g/L was produced. Recently, Bruder et al. (2019) revealed that the engineered Y. lipolytica is able to produce odd-numbered alkanes and alkenes (mainly C15 and C17), by the expression of light-driven oxidase. Interestingly, using the lighting bioreactors, the titer of alkenes at 58.7 mg/L was first reached in this research.
Biochemicals
γ-decalactone, a well-known aroma compound, is mainly synthesized via β-oxidation. Previously, we have summarized in detail the γ-decalactone production by Y. lipolytica (Liu et al., 2015). Recently, using the immobilized culture technology, the maximum production of γ-decalactone reached 8.37 g/L by Y. lipolytica strain on bacterial cellulose-alginate carriers (Zhang et al., 2020). Additionally, using a one-pot biotransformation process containing whole Y. lipolytica cells, the highest production of δ-decalactone at 58.7 mg/L was first performed (Kang et al., 2016).
HFAs, as valuable building blocks, can be synthesized by the biotransformation of fatty acids via the terminal carbon oxygenation (Seo et al., 2015). To date, the microbial production of ω-HFAs by the engineered E. coli has received specific progress (Kim and Park, 2019). Using Y. lipolytica as a promising workhorse, an engineered strain capable of synthesizing ω-hydroxy dodecanoic acid was constructed, through the deletion of acyl-CoA oxidase-coding genes (POX 1–6), fatty alcohol oxidase gene (FAO1), and alcohol dehydrogenase genes (ADH 1–8) (Gatter et al., 2014). Recently, Rigouin et al. (2019) showed that the engineered Y. lipolytica is able to produce polyhydroxyalkanoates composed of 3-HFAs, using methyl myristate as precursor.
DCAs are also important intermediates in the industrial field. At present, the microbial production of DCAs, as an alternative method, are gaining interests (Huf et al., 2011; Ledesma-Amaro and Nicaud, 2016b; Werner and Zibek, 2017). Y. lipolytica can produce DCAs via alkane degradation (Nicaud et al., 2006). Previously, researchers have shown that the engineered Y. lipolytica can produce dioic acids (Smit et al., 2005; Nicaud et al., 2006). In particular, Gatter et al. (2014) showed that the overexpression of FAO1 leads to an improved production of dodecane dioic acid at 11 g/L. Recently, using the in silico model-based metabolic engineering strategies, the metabolic flux toward DCAs production was obviously increased in Y. lipolytica (Mishra et al., 2018).
Hexanal, one of C-6 aldehydes with green odor, can be synthesized via the degradation from LA using lipoxygenase and hydroperoxide lyase. Previously, using Y. lipolytica as a host, Bourel et al. (2004) showed that hexanal is produced by expressing of fatty acid hydroperoxide lyase. Further, Santiago-Gómez et al. (2009) reported the effect of oxido-reduction environment on hexanal production. Interestingly, in this research, under the optimized conditions, the highest titer of hexanal at 600 mg/L was produced by the engineered Y. lipolytica.
In addition, cyclopropane fatty acids (CFAs), as good unusual fatty acid candidates, were produced by the engineered Y. lipolytica (Czerwiec et al., 2019). In this research, by expressing genes from various organisms and optimizing the expression level of CFAs synthase and fed-batch fermentation, it was shown that CFAs at 2319 mg/L (mainly C17:0 and C19:0 cyclopropanated form) are finally synthesized in the strain JMY 6068. Compared with E. coli and S. cerevisiae, the fatty acid derivatives produced by Y. lipolytica are more abundant (Table 3).
TABLE 3.
Comparison of the productivity of fatty acid-derived biofuels between E. coli, S. cerevisiae, and Y. lipolytica.
|
E. coli |
S. cerevisiae |
Y. lipolytica |
||||
| Titer | References | Titer | References | Titer | References | |
| Fatty alcohols | 1.8 g/L | Mehrer et al., 2018 | 6.0 g/L (Fed-batch) | d’Espaux et al., 2017 | 2.15 g/L (in a 3-L bioreactor) | Xu et al., 2016 |
| FAEE | 1.5 g/L (minimal medium) | Zhang F. et al., 2012 | 0.005 g/L | Runguphan and Keasling, 2014 | 1.18 g/L (containing 5 vol% ethanol) | Gao et al., 2018 |
| FFAs | 2.1 g/L (modified MOPS minimal medium) | Kim and Gonzalez, 2018 | 33.4 g/L (Fed-batch) | Yu et al., 2018 | 9.67 g/L (in a 3-L bioreactor) | Xu et al., 2016 |
| Alkanes | 0.426 g/L | Fatma et al., 2018 | 0.003 g/L (Delft minimal medium) | Zhu et al., 2017 | 58.7 mg/L (Fed-batch) | Bruder et al., 2019 |
| γ-decalactone | ND | ND | Increase by 11% | Rong et al., 2017 | 8.37 g/L | Zhang et al., 2020 |
| HFAs | 275 mg/L | He et al., 2019 | 347 mg/L (Fed-batch) | Liu J. J. et al., 2019 | 7.9 g/L | Gatter et al., 2014 |
| DCAs | ND | Wang F. et al., 2020 | 92.5 g/L (Fed-batch) | Lee et al., 2018 | 11 g/L | Gatter et al., 2014 |
| Hexanal | ND | ND | ND | ND | increased by 84.1% | Aziz et al., 2016 |
| CFA | ND | Guangqi et al., 2010 | ND | Kochan et al., 2019 | 2319 mg/L | Czerwiec et al., 2019 |
ND, not determine.
Conclusion and Future Perspectives
Y. lipolytica is a promising workhorse gaining great attention. Currently, the advance of metabolic engineering and synthetic biology enables Y. lipolytica to produce various value-added chemicals with different substrates and metabolic engineering strategies, including the design and construction of synthetic pathways, regulation of endogenous genes, and optimization of the fermentation process. However, several challenges remain in limiting the wide applications of Y. lipolytica.
When developing and optimizing Y. lipolytica for improving the production of value-added chemicals, the whole bioprocess, including the upstream of strain development and bioproducts production, the midstream of scale-up fermentation, and the downstream of recovery and purification, is needed to be considered first. Ko et al. (2020) showed that systems metabolic engineering, integrating systems biology, synthetic biology, and evolutionary engineering can enable microbial strains to efficiently produce chemicals. Therefore, systems metabolic engineering can be further applied to better manipulate the engineered Y. lipolytica to synthesize the desired bioproducts. Meanwhile, to optimize cell metabolism, such as reducing the negative effects of intermediate accumulation and metabolic perturbations, the dynamic metabolic engineering capable of tuning the cell growth and bioproducts formation is becoming a promising approach to better engineer the host strain (Xu, 2018). Moreover, due to the limits of dimorphic nature, cellular engineering and bioprocess engineering can be used to improve the yield of products at the industrial scale (Soong et al., 2019). Additionally, to reduce the cost of bioprocess, other low-value carbon sources, especially single-carbon substrates, will be utilized and converted to valuable fatty acid-based bioproducts by metabolic engineering Y. lipolytica. Conclusively, the application of Y. lipolytica for fatty acid-based chemicals production shows a great promise for researchers working in this field.
Author Contributions
HL conceived the outline and revised the manuscript. YT finalized the topic of this review, and all authors wrote the manuscript. All authors read and approved the final manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Key R&D Program of China (No. 2019YFC1604903), the National Natural Science Foundation of China (No. 21808052), the China Postdoctoral Science Foundation (No. 2019TQ0088), the Scientific Research Foundation of Hunan Provincial Education Department (Nos. 18B090 and 18K039), and the Double First-Class Construction Project of Hunan Agricultural University (No. SYL201802002).
References
- Abdel-Mawgoud A. M., Markham K. A., Palmer C. M., Liu N., Stephanopoulos G., Alper H. S. (2018). Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 50 192–208. 10.1016/j.ymben.2018.07.016 [DOI] [PubMed] [Google Scholar]
- Abghari A., Chen S. L. (2014). Yarrowia lipolytica as an oleaginous cell factory platform for production of fatty acid-based biofuel and bioproducts. Front. Energy Res. 2:21 10.3389/fenrg.2014.00021 [DOI] [Google Scholar]
- Aggelis G., Papadiotis G., Komaitis M. (1997). Microbial fatty acid specificity. Folia Microbiol. 42 117–120. 10.1007/BF02898718 [DOI] [PubMed] [Google Scholar]
- Aguedo M., Gomes N., Garcia E. E., Wache Y., Mota M., Teixeira J. A., et al. (2005). Decalactone production by Yarrowia lipolytica under increased O2 transfer rates. Biotechnol. Lett. 27 1617–1621. 10.1007/s10529-005-2517-z [DOI] [PubMed] [Google Scholar]
- Aziz M., St-Louis R., Husson F., Kermasha S. (2016). Selected dehydrogenases in Yarrowia lipolytica JMY 861: their role in the synthesis of flavor compounds. Biosci. Biotechnol. Biochem. 80 2184–2191. 10.1080/09168451.2016.1214531 [DOI] [PubMed] [Google Scholar]
- Bellou S., Triantaphyllidou I. E., Aggeli D., Elazzazy A. M., Baeshen M. N., Aggelis G. (2016). Microbial oils as food additives: recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 37 24–35. 10.1016/j.copbio.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Beopoulos A., Cescut J., Haddouche R., Uribelarrea J.-L., Molina-Jouve C., Nicaud J.-M. (2009). Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 48 375–387. 10.1016/j.plipres.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Beopoulos A., Nicaud J. M., Gaillardin C. (2011). An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 90 1193–1206. 10.1007/s00253-011-3212-8 [DOI] [PubMed] [Google Scholar]
- Beopoulos A., Verbeke J., Bordes F., Guicherd M., Bressy M., Marty A., et al. (2014). Metabolic engineering for ricinoleic acid production in the oleaginous yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 98 251–262. 10.1007/s00253-013-5295-x [DOI] [PubMed] [Google Scholar]
- Blazeck J., Liu L., Knight R., Alper H. S. (2013). Heterologous production of pentane in the oleaginous yeast Yarrowia lipolytica. J. Biotechnol. 165 184–194. 10.1016/j.jbiotec.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Bourel G., Nicaud J. M., Nthangeni B., Santiago-Gomez P., Belin J. M., Husson F. (2004). Fatty acid hydroperoxide lyase of green bell pepper: cloning in Yarrowia lipolytica and biogenesis of volatile aldehydes. Enzyme Microb. Technol. 35 293–299. 10.1016/j.enzmictec.2003.12.014 [DOI] [Google Scholar]
- Braga A., Belo I. (2013). Immobilization of Yarrowia lipolytica for aroma production from castor oil. Appl. Biochem. Biotechnol. 169 2202–2211. 10.1007/s12010-013-0131-4 [DOI] [PubMed] [Google Scholar]
- Braga A., Belo I. (2014). Production of γ-decalactone by Yarrowia lipolytica: insights into experimental conditions and operating mode optimization. J. Chem. Technol. Biotechnol. 90 559–565. 10.1002/jctb.4349 [DOI] [Google Scholar]
- Bruder S., Moldenhauer E. J., Lemke R. D., Ledesma-Amaro R., Kabisch J. (2019). Drop-in biofuel production using fatty acid photodecarboxylase from Chlorella variabilis in the oleaginous yeast Yarrowia lipolytica. Biotechnol. Biofuels 12:202. 10.1186/s13068-019-1542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. J., Lee S. Y. (2013). Microbial production of short-chain alkanes. Nature 502 571–574. 10.1038/nature12536 [DOI] [PubMed] [Google Scholar]
- Chuang L. T., Chen D. C., Nicaud J. M., Madzak C., Chen Y. H., Huang Y. S. (2010). Co-expression of heterologous desaturase genes in Yarrowia lipolytica. N. Biotechnol. 27 277–282. 10.1016/j.nbt.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Cordova L. T., Alper H. S. (2018). Production of α-linolenic acid in Yarrowia lipolytica using low-temperature fermentation. Appl. Microbiol. Biotechnol. 102 8809–8816. 10.1007/s00253-018-9349-y [DOI] [PubMed] [Google Scholar]
- Czerwiec Q., Idrissitaghki A., Imatoukene N., Nonus M., Thomasset B., Nicaud J. M., et al. (2019). Optimization of cyclopropane fatty acids production in Yarrowia lipolytica. Yeast 36 143–151. 10.1002/yea.3379 [DOI] [PubMed] [Google Scholar]
- Darvishi F., Ariana M., Marella E. R., Borodina I. (2018). Advances in synthetic biology of oleaginous yeast Yarrowia lipolytica for producing non-native chemicals. Appl. Microbiol. Biotechnol. 102 5925–5938. 10.1007/s00253-018-9099-x [DOI] [PubMed] [Google Scholar]
- d’Espaux L., Ghosh A., Runguphan W., Wehrs M., Xu F., Konzock O., et al. (2017). Engineering high-level production of fatty alcohols by Saccharomyces cerevisiae from lignocellulosic feedstocks. Metab. Eng. 42 115–125. 10.1016/j.ymben.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Dulermo R., Gamboa-Melendez H., Ledesma-Amaro R., Thevenieau F., Nicaud J. M. (2015). Unraveling fatty acid transport and activation mechanisms in Yarrowia lipolytica. Biochim. Biophys. Acta 1851 1202–1217. 10.1016/j.bbalip.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Dulermo T., Nicaud J. M. (2011). Involvement of the G3P shuttle and β-oxidation pathway in the control of TAG synthesis and lipid accumulation in Yarrowia lipolytica. Metab. Eng. 13 482–491. 10.1016/j.ymben.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Ethier S., Woisard K., Vaughan D., Wen Z. (2011). Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 102 88–93. 10.1016/j.biortech.2010.05.021 [DOI] [PubMed] [Google Scholar]
- Fatma Z., Hartman H., Poolman M. G., Fell D. A., Srivastava S., Shakeel T., et al. (2018). Model-assisted metabolic engineering of Escherichia coli for long chain alkane and alcohol production. Metab. Eng. 46 1–12. 10.1016/j.ymben.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Fickers P., Benetti P. H., Wache Y., Marty A., Mauersberger S., Smit M. S., et al. (2005). Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 5 527–543. 10.1016/j.femsyr.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Gao Q., Cao X., Huang Y. Y., Yang J. L., Chen J., Wei L. J., et al. (2018). Overproduction of fatty acid ethyl esters by the oleaginous yeast Yarrowia lipolytica through metabolic engineering and process optimization. ACS. Synth. Biol. 7 1371–1380. 10.1021/acssynbio.7b00453 [DOI] [PubMed] [Google Scholar]
- Gatter M., Forster A., Bar K., Winter M., Otto C., Petzsch P., et al. (2014). A newly identified fatty alcohol oxidase gene is mainly responsible for the oxidation of long-chain omega-hydroxy fatty acids in Yarrowia lipolytica. FEMS Yeast Res. 14 858–872. 10.1111/1567-1364.12176 [DOI] [PubMed] [Google Scholar]
- Gemperlein K., Dietrich D., Kohlstedt M., Zipf G., Bernauer H. S., Wittmann C., et al. (2019). Polyunsaturated fatty acid production by Yarrowia lipolytica employing designed myxobacterial PUFA synthases. Nat. Commun. 10:4055. 10.1038/s41467-019-12025-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes N., Aguedo M., Teixeira J., Belo I. (2007). Oxygen mass transfer in a biphasic medium: influence on the biotransformation of methyl ricinoleate into γ-decalactone by the yeast Yarrowia lipolytica. Biochem. Eng. J. 35 380–386. 10.1016/j.bej.2007.02.002 [DOI] [Google Scholar]
- Gomes N., Teixeira J. A., Belo I. (2010). The use of methyl ricinoleate in lactone production by Yarrowia lipolytica: aspects of bioprocess operation that influence the overall performance. Biocatal. Biotransformation 28 227–234. 10.3109/10242422.2010.493208 [DOI] [Google Scholar]
- Gomes N., Teixeira J. A., Belo I. (2012). Fed-batch versus batch cultures of Yarrowia lipolytica for gamma-decalactone production from methyl ricinoleate. Biotechnol. Lett. 34 649–654. 10.1007/s10529-011-0824-0 [DOI] [PubMed] [Google Scholar]
- Guangqi E., Lesage D., Ploux O. (2010). Insight into the reaction mechanism of the Escherichia coli cyclopropane fatty acid synthase: isotope exchange and kinetic isotope effects. Biochimie 92 1454–1457. 10.1016/j.biochi.2010.05.019 [DOI] [PubMed] [Google Scholar]
- Guo Z. P., Robin J., Duquesne S., O’Donohue M. J., Marty A., Bordes F. (2018). Developing cellulolytic Yarrowia lipolytica as a platform for the production of valuable products in consolidated bioprocessing of cellulose. Biotechnol. Biofuels 11:141. 10.1186/s13068-018-1144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer S. K., Avalos J. L. (2017). Harnessing yeast organelles for metabolic engineering. Nat. Chem. Biol. 13 823–832. 10.1038/nchembio.2429 [DOI] [PubMed] [Google Scholar]
- He Q., Bennett G. N., San K. Y., Wu H. (2019). Biosynthesis of medium-chain omega-hydroxy fatty acids by AlkBGT of Pseudomonas putida GPo1 with native FadL in engineered Escherichia coli. Front. Bioeng. Biotechnol. 7:273. 10.3389/fbioe.2019.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holic R., Yazawa H., Kumagai H., Uemura H. (2012). Engineered high content of ricinoleic acid in fission yeast Schizosaccharomyces pombe. Appl. Microbiol. Biotechnol. 95 179–187. 10.1007/s00253-012-3959-6 [DOI] [PubMed] [Google Scholar]
- Huf S., Krügener S., Hirth T., Rupp S., Zibek S. (2011). Biotechnological synthesis of long-chain dicarboxylic acids as building blocks for polymers. Eur. J. Lipid Sci. Technol. 113 548–561. 10.1002/ejlt.201000112 [DOI] [Google Scholar]
- Imatoukene N., Verbeke J., Beopoulos A., Idrissi Taghki A., Thomasset B., Sarde C. O., et al. (2017). A metabolic engineering strategy for producing conjugated linoleic acids using the oleaginous yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 101 4605–4616. 10.1007/s00253-017-8240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawed K., Yazdani S. S., Koffas M. A. G. (2019). Advances in the development and application of microbial consortia for metabolic engineering. Metab. Eng. Commun. 9:e00095. 10.1016/j.mec.2019.e00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X. J., Huang H. (2019). Engineering microbes to produce polyunsaturated fatty acids. Trends Biotechnol. 37 344–346. 10.1016/j.tibtech.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Ji X.-J., Ledesma-Amaro R. (2020). Microbial lipid biotechnology to produce polyunsaturated fatty acids. Trends Biotechnol. 38 832–834. 10.1016/j.tibtech.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Kang W. R., Seo M. J., An J. U., Shin K. C., Oh D. K. (2016). Production of delta-decalactone from linoleic acid via 13-hydroxy-9(Z)-octadecenoic acid intermediate by one-pot reaction using linoleate 13-hydratase and whole Yarrowia lipolytica cells. Biotechnol. Lett. 38 817–823. 10.1007/s10529-016-2041-3 [DOI] [PubMed] [Google Scholar]
- Kim S., Gonzalez R. (2018). Selective production of decanoic acid from iterative reversal of β-oxidation pathway. Biotechnol. Bioeng. 115 1311–1320. 10.1002/bit.26540 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Park Y. C. (2019). Biosynthesis of ω-hydroxy fatty acids and related chemicals from natural fatty acids by recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 103 191–199. 10.1007/s00253-018-9503-6 [DOI] [PubMed] [Google Scholar]
- Ko Y. S., Kim J. W., Lee J. A., Han T., Kim G. B., Park J. E., et al. (2020). Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production. Chem. Soc. Rev. 49 4615–4636. 10.1039/d0cs00155d [DOI] [PubMed] [Google Scholar]
- Kochan K., Peng H., Gwee E. S. H., Izgorodina E., Haritos V., Wood B. R. (2019). Raman spectroscopy as a tool for tracking cyclopropane fatty acids in genetically engineered Saccharomyces cerevisiae. Anal. 144 901–912. 10.1039/c8an01477a [DOI] [PubMed] [Google Scholar]
- Kolouchová I., Schreiberová O., Sigler K., Masák J., Řezanka T. (2015). Biotransformation of volatile fatty acids by oleaginous and non-oleaginous yeast species. FEMS Yeast Res. 15:fov076. 10.1093/femsyr/fov076 [DOI] [PubMed] [Google Scholar]
- Larroude M., Rossignol T., Nicaud J. M., Ledesma-Amaro R. (2018). Synthetic biology tools for engineering Yarrowia lipolytica. Biotechnol. Adv. 36 2150–2164. 10.1016/j.biotechadv.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar Z., Liu N., Stephanopoulos G. (2018). Holistic approaches in lipid production by Yarrowia lipolytica. Trends Biotechnol. 36 1157–1170. 10.1016/j.tibtech.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Ledesma-Amaro R., Dulermo R., Niehus X., Nicaud J. M. (2016). Combining metabolic engineering and process optimization to improve production and secretion of fatty acids. Metab. Eng. 38 38–46. 10.1016/j.ymben.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Ledesma-Amaro R., Nicaud J. M. (2016a). Metabolic engineering for expanding the substrate range of Yarrowia lipolytica. Trends Biotechnol. 34 798–809. 10.1016/j.tibtech.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Ledesma-Amaro R., Nicaud J. M. (2016b). Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog. Lipid Res. 61 40–50. 10.1016/j.plipres.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Lee H., Han C., Lee H.-W., Park G., Jeon W., Ahn J., et al. (2018). Development of a promising microbial platform for the production of dicarboxylic acids from biorenewable resources. Biotechnol. Biofuels 11:310. 10.1186/s13068-018-1310-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levering J., Broddrick J., Zengler K. (2015). Engineering of oleaginous organisms for lipid production. Curr. Opin. Biotechnol. 36 32–39. 10.1016/j.copbio.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Li J., Jiang Z., Wu H., Long L., Jiang Y., Zhang L. (2009). Improving the recycling and storage stability of enzyme by encapsulation in mesoporous CaCO3-alginate composite gel. Composites Sci. Technol. 69 539–544. 10.1016/j.compscitech.2008.11.017 [DOI] [Google Scholar]
- Li S.-Y., Srivastava R., Suib S. L., Li Y., Parnas R. S. (2011). Performance of batch, fed-batch, and continuous A-B-E fermentation with pH-control. Bioresour. Technol. 102 4241–4250. 10.1016/j.biortech.2010.12.078 [DOI] [PubMed] [Google Scholar]
- Li X., Hu B., Li H., You B. (2019). Application of artificial intelligence in the diagnosis of multiple primary lung cancer. Thorac. Cancer 10 2168–2174. 10.1111/1759-7714.13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. H., Ji X. J., Huang H. (2015). Biotechnological applications of Yarrowia lipolytica: past, present and future. Biotechnol. Adv. 33 1522–1546. 10.1016/j.biotechadv.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Liu H. H., Madzak C., Sun M. L., Ren L. J., Song P., Huang H., et al. (2017a). Engineering Yarrowia lipolytica for arachidonic acid production through rapid assembly of metabolic pathway. Biochem. Eng. J. 119 52–58. 10.1016/j.bej.2016.12.004 [DOI] [Google Scholar]
- Liu H. H., Zeng S. Y., Shi T. Q., Ding Y., Ren L. J., Song P., et al. (2017b). A Yarrowia lipolytica strain engineered for arachidonic acid production counteracts metabolic burden by redirecting carbon flux towards intracellular fatty acid accumulation at the expense of organic acids secretion. Biochem. Eng. J. 128 201–209. 10.1016/j.bej.2017.10.007 [DOI] [Google Scholar]
- Liu H. H., Wang C., Lu X. Y., Huang H., Tian Y., Ji X. J. (2019). Improved production of arachidonic acid by combined pathway engineering and synthetic enzyme fusion in Yarrowia lipolytica. J. Agric. Food Chem. 67 9851–9857. 10.1021/acs.jafc.9b03727 [DOI] [PubMed] [Google Scholar]
- Liu J. J., Zhang C. B., Lu W. Y. (2019). Biosynthesis of long-chain omega-hydroxy fatty acids by engineered Saccharomyces cerevisiae. J. Agric. Food Chem 67 4545–4552. 10.1021/acs.jafc.9b00109 [DOI] [PubMed] [Google Scholar]
- Ma J., Gu Y., Marsafari M., Xu P. (2020). Synthetic biology, systems biology, and metabolic engineering of Yarrowia lipolytica toward a sustainable biorefinery platform. J. Ind. Microbiol. Biotechnol. 47 845–862. 10.1007/s10295-020-02290-8 [DOI] [PubMed] [Google Scholar]
- Ma Y. R., Wang K. F., Wang W. J., Ding Y., Shi T. Q., Huang H., et al. (2019). Advances in the metabolic engineering of Yarrowia lipolytica for the production of terpenoids. Bioresour. Technol. 281 449–456. 10.1016/j.biortech.2019.02.116 [DOI] [PubMed] [Google Scholar]
- Macario A., Moliner M., Corma A., Giordano G. (2009). Increasing stability and productivity of lipase enzyme by encapsulation in a porous organic-inorganic system. Microporous Mesoporous Mater. 118 334–340. 10.1016/j.micromeso.2008.09.003 [DOI] [Google Scholar]
- Madzak C. (2018). Engineering Yarrowia lipolytica for use in biotechnological applications: a review of major achievements and recent innovations. Mol. Biotechnol. 60 621–635. 10.1007/s12033-018-0093-4 [DOI] [PubMed] [Google Scholar]
- Markham K. A., Alper H. S. (2018). Engineering Yarrowia lipolytica for the production of cyclopropanated fatty acids. J. Ind. Microbiol. Biotechnol. 45 881–888. 10.1007/s10295-018-2067-8 [DOI] [PubMed] [Google Scholar]
- Markham K. A., Palmer C. M., Chwatko M., Wagner J. M., Murray C., Vazquez S., et al. (2018). Rewiring Yarrowia lipolytica toward triacetic acid lactone for materials generation. Proc. Natl. Acad. Sci. U.S.A. 115 2096–2101. 10.1073/pnas.1721203115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil B. A., Stuart D. T. (2018). Lipomyces starkeyi: an emerging cell factory for production of lipids, oleochemicals and biotechnology applications. World J. Microbiol. Biotechnol. 34:147. 10.1007/s11274-018-2532-6 [DOI] [PubMed] [Google Scholar]
- Meesapyodsuk D., Qiu X. (2008). An oleate hydroxylase from the fungus Claviceps purpurea: cloning, functional analysis, and expression in Arabidopsis. Plant Physiol. 147 1325–1333. 10.1104/pp.108.117168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrer C. R., Incha M. R., Politz M. C., Pfleger B. F. (2018). Anaerobic production of medium-chain fatty alcohols via a β-reduction pathway. Metab. Eng. 48 63–71. 10.1016/j.ymben.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. K., Alper H. S. (2019). Yarrowia lipolytica: more than an oleaginous workhorse. Appl. Microbiol. Biotechnol. 103 9251–9262. 10.1007/s00253-019-10200-x [DOI] [PubMed] [Google Scholar]
- Mirończuk A. M., Kosiorowska K. E., Biegalska A., Rakicka-Pustułka M., Szczepañczyk M., Dobrowolski A. (2019). Heterologous overexpression of bacterial hemoglobin VHb improves erythritol biosynthesis by yeast Yarrowia lipolytica. Microb. Cell Fact. 18:176. 10.1186/s12934-019-1231-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P., Lee N. R., Lakshmanan M., Kim M., Kim B. G., Lee D. Y. (2018). Genome-scale model-driven strain design for dicarboxylic acid production in Yarrowia lipolytica. BMC Syst. Biol. 12:12. 10.1186/s12918-018-0542-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi H., Asadollahi M. A., Nahvi I. (2013). Improved γ-decalactone production from castor oil by fed-batch cultivation of Yarrowia lipolytica. Biocatal. Agric. Biotechnol. 2 64–68. 10.1016/j.bcab.2012.11.001 [DOI] [Google Scholar]
- Nawabi P., Bauer S., Kyrpides N., Lykidis A. (2011). Engineering Escherichia coli for biodiesel production utilizing a bacterial fatty acid methyltransferase. Appl. Environ. Microbiol. 77 8052–8061. 10.1128/AEM.05046-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T.-K., Yu A.-Q., Ling H., Pratomo Juwono N. K., Choi W. J., Leong S. S. J., et al. (2019). Engineering Yarrowia lipolytica towards food waste bioremediation: production of fatty acid ethyl esters from vegetable cooking oil. J. Biosci. Bioeng. 129 31–40. 10.1016/j.jbiosc.2019.06.009 [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Thevenieau F., Dall M. T. L., Marchal R. (2006). Production of Dicarboxylic Acids by Improved Mutant Strains of Yarrowia lipolytica. European Patent No 1828392B1. Paris: European patent office. [Google Scholar]
- Pagot Y., Endrizzi A., Nicaud J. M., Belin J. M. (1997). Utilization of an auxotrophic strain of the yeast Yarrowia lipolytica. Lett. Appl. Microbiol. 25 113–116. [DOI] [PubMed] [Google Scholar]
- Park Y. K., Dulermo T., Ledesma-Amaro R., Nicaud J. M. (2018a). Optimization of odd chain fatty acid production by Yarrowia lipolytica. Biotechnol. Bioeng. 11:158. 10.1186/s13068-018-1154-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. K., Nicaud J. M., Ledesma-Amaro R. (2018b). The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications. Trends Biotechnol. 36 304–317. 10.1016/j.tibtech.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Park Y. K., Ledesma-Amaro R., Nicaud J. M. (2020). De novo biosynthesis of odd-chain fatty acids in Yarrowia lipolytica enabled by modular pathway engineering. Front. Bioeng. Biotechnol. 7:484. 10.3389/fbioe.2019.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Andrade D., Carvalho B. F., Schwan R. F., Dias D. R. (2017). Production of gamma-decalactone by yeast strains under different conditions. Food Technol. Biotechnol. 55 225–230. 10.17113/ftb.55.02.17.5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst K. V., Schulte L. R., Durrett T. P., Rezac M. E., Vadlani P. V. (2016). Oleaginous yeast: a value-added platform for renewable oils. Crit. Rev. Biotechnol. 36 942–955. 10.3109/07388551.2015.1064855 [DOI] [PubMed] [Google Scholar]
- Qiao K., Wasylenko T. M., Zhou K., Xu P., Stephanopoulos G. (2017). Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat. Biotechnol. 35 173–177. 10.1038/nbt.3763 [DOI] [PubMed] [Google Scholar]
- Řezanka T., Sigler K. (2009). Odd-numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. Prog. Lipid Res. 48 206–238. 10.1016/j.plipres.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Rigouin C., Lajus S., Ocando C., Borsenberger V., Nicaud J. M., Marty A., et al. (2019). Production and characterization of two medium-chain-length polydroxyalkanoates by engineered strains of Yarrowia lipolytica. Microb. Cell Fact. 18:99. 10.1186/s12934-019-1140-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Rodriguez C. E., Munoz-Tamayo R., Bideaux C., Gorret N., Guillouet S. E., Molina-Jouve C., et al. (2018). Modeling and optimization of lipid accumulation by Yarrowia lipolytica from glucose under nitrogen depletion conditions. Biotechnol. Bioeng. 115 1137–1151. 10.1002/bit.26537 [DOI] [PubMed] [Google Scholar]
- Rong S., Yang S., Li Q., Cai B., Guan S., Wang J., et al. (2017). Improvement of γ-decalactone production by stimulating the import of ricinoleic acid and suppressing the degradation of γ-decalactone in Saccharomyces cerevisiae. Biocatal. Biotransformation 35 96–102. 10.1080/10242422.2017.1289182 [DOI] [Google Scholar]
- Runguphan W., Keasling J. D. (2014). Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid-derived biofuels and chemicals. Metab. Eng. 21 103–113. 10.1016/j.ymben.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Rutter C. D., Rao C. V. (2016). Production of 1-decanol by metabolically engineered Yarrowia lipolytica. Metab. Eng. 38 139–147. 10.1016/j.ymben.2016.07.011 [DOI] [PubMed] [Google Scholar]
- Santiago-Gómez M. P., Thanh H. T., De Coninck J., Cachon R., Kermasha S., Belin J.-M., et al. (2009). Modeling hexanal production in oxido-reducing conditions by the yeast Yarrowia lipolytica. Process Biochem. 44 1013–1018. 10.1016/j.procbio.2009.04.028 [DOI] [Google Scholar]
- Seip J., Jackson R., He H., Zhu Q., Hong S. P. (2013). Snf1 is a regulator of lipid accumulation in Yarrowia lipolytica. Appl. Environ. Microbiol. 79 7360–7370. 10.1128/AEM.02079-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J. H., Lee S. M., Lee J., Park J. B. (2015). Adding value to plant oils and fatty acids: biological transformation of fatty acids into ω-hydroxycarboxylic, α,ω-dicarboxylic, and ω-aminocarboxylic acids. J. Biotechnol. 216 158–166. 10.1016/j.jbiotec.2015.10.024 [DOI] [PubMed] [Google Scholar]
- Smit M. S., Mokgoro M. M., Setati E., Nicaud J. M. (2005). α,ω-Dicarboxylic acid accumulation by acyl-CoA oxidase deficient mutants of Yarrowia lipolytica. Biotechnol. Lett. 27 859–864. 10.1007/s10529-005-6719-1 [DOI] [PubMed] [Google Scholar]
- Soares G. P. A., Souza K. S. T., Vilela L. F., Schwan R. F., Dias D. R. (2017). γ-decalactone production by Yarrowia lipolytica and Lindnera saturnus in crude glycerol. Prep. Biochem. Biotechnol. 47 633–637. 10.1080/10826068.2017.1286601 [DOI] [PubMed] [Google Scholar]
- Soong Y. H. V., Liu N., Yoon S., Lawton C., Xie D. (2019). Cellular and metabolic engineering of oleaginous yeast Yarrowia lipolytica for bioconversion of hydrophobic substrates into high-value products. Eng. Life Sci. 19 423–443. 10.1002/elsc.201800147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnuolo M., Shabbir Hussain M., Gambill L., Blenner M. (2018). Alternative substrate metabolism in Yarrowia lipolytica. Front. Microbiol. 9:1077. 10.3389/fmicb.2018.01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen E. J., Kang Y., Bokinsky G., Hu Z., Schirmer A., McClure A., et al. (2010). Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463 559–562. 10.1038/nature08721 [DOI] [PubMed] [Google Scholar]
- Suen Y. L., Tang H., Huang J., Chen F. (2014). Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp by the expression of Vitreoscilla Hemoglobin. J. Agric. Food Chem. 62 12392–12398. 10.1021/jf5048578 [DOI] [PubMed] [Google Scholar]
- Sun M. L., Madzak C., Liu H. H., Song P., Ren L. J., Huang H., et al. (2017). Engineering Yarrowia lipolytica for efficient γ-linolenic acid production. Biochem. Eng. J. 117 172–180. 10.1016/j.bej.2016.10.014 [DOI] [Google Scholar]
- Wache Y., Aguedo M., Choquet A., Gatfield I. L., Nicaud J. M., Belin J. M. (2001). Role of β-oxidation enzymes in γ-decalactone production by the yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 67 5700–5704. 10.1128/AEM.67.12.5700-5704.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waché Y., Aguedoa M., LeDall M. T., Nicaud J. M., Belin J. M. (2002). Optimization of Yarrowia lipolytica’s β-oxidation pathway for γ-decalactone production. J. Mol. Catal. 19–20 347–351. [Google Scholar]
- Wang F., Zhao J., Li Q., Yang J., Li R., Min J., et al. (2020). One-pot biocatalytic route from cycloalkanes to α,ω-dicarboxylic acids by designed Escherichia coli consortia. Nat. Commun. 11:5035 10.1038/s41467-020-18833-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Xiong X., Ghogare R., Wang P., Meng Y., Chen S. (2016). Exploring fatty alcohol-producing capability of Yarrowia lipolytica. Biotechnol. Biofuels 9:107. 10.1186/s13068-016-0512-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ledesma-Amaro R., Wei Y., Ji B., Ji X. J. (2020). Metabolic engineering for increased lipid accumulation in Yarrowia lipolytica-a review. Bioresour. Technol. 313:123707 10.1016/j.biortech.2020.123707 [DOI] [PubMed] [Google Scholar]
- Wang X., Xia Q., Wang F., Zhang Y., Li X. (2019). Modulating heterologous pathways and optimizing culture conditions for biosynthesis of trans-10, cis-12 conjugated linoleic acid in Yarrowia lipolytica. Molecules 24:1753. 10.3390/molecules24091753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylenko T. M., Ahn W. S., Stephanopoulos G. (2015). The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab. Eng. 30 27–39. 10.1016/j.ymben.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Werner N., Zibek S. (2017). Biotechnological production of bio-based long-chain dicarboxylic acids with oleogenious yeasts. World J. Microbiol. Biotechnol. 33:194. 10.1007/s11274-017-2360-0 [DOI] [PubMed] [Google Scholar]
- Xie D. (2017). Integrating cellular and bioprocess engineering in the non-conventional yeast Yarrowia lipolytica for biodiesel production: a review. Front. Bioeng. Biotechnol. 5:65. 10.3389/fbioe.2017.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D., Jackson E. N., Zhu Q. (2015). Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered Yarrowia lipolytica: from fundamental research to commercial production. Appl. Microbiol. Biotechnol. 99 1599–1610. 10.1007/s00253-014-6318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D., Miller E., Sharpe P., Jackson E., Zhu Q. (2017). Omega-3 production by fermentation of Yarrowia lipolytica: from fed-batch to continuous. Biotechnol. Bioeng. 114 798–812. 10.1002/bit.26216 [DOI] [PubMed] [Google Scholar]
- Xu P. (2018). Production of chemicals using dynamic control of metabolic fluxes. Curr. Opin. Biotechnol. 53 12–19. 10.1016/j.copbio.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Xu P., Qiao K., Ahn W. S., Stephanopoulos G. (2016). Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc. Natl. Acad. Sci. U.S.A. 113 10848–10853. 10.1073/pnas.1607295113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Qian S. Y. (2014). Anti-cancer activities of omega-6 polyunsaturated fatty acids. Biomed. J. 37 112–119. 10.4103/2319-4170.131378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z., Sharpe P. L., Hong S.-P., Yadav N. S., Xie D., Short D. R., et al. (2013). Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat. Biotechnol. 31 734–740. 10.1038/nbt.2622 [DOI] [PubMed] [Google Scholar]
- Yan Q., Pfleger B. F. (2020). Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng. 58 35–46. 10.1016/j.ymben.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Yu A., Zhao Y., Li J., Li S., Pang Y., Zhao Y., et al. (2020). Sustainable production of FAEE biodiesel using the oleaginous yeast Yarrowia lipolytica. Microbiologyopen 9:e1051 10.1002/mbo3.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. O., Jung J., Kim S. W., Park C. H., Han S. O. (2012). Synthesis of FAEEs from glycerol in engineered Saccharomyces cerevisiae using endogenously produced ethanol by heterologous expression of an unspecific bacterial acyltransferase. Biotechnol. Bioeng. 109 110–115. 10.1002/bit.23311 [DOI] [PubMed] [Google Scholar]
- Yu T., Zhou Y. J., Huang M., Liu Q., Pereira R., David F., et al. (2018). Reprogramming yeast metabolism from alcoholic fermentation to lipogenesis. Cell 174 1549.e14–1558.e14. 10.1016/j.cell.2018.07.013 [DOI] [PubMed] [Google Scholar]
- Yuzbasheva E. Y., Mostova E. B., Andreeva N. I., Yuzbashev T. V., Fedorov A. S., Konova I. A., et al. (2018). A metabolic engineering strategy for producing free fatty acids by the Yarrowia lipolytica yeast based on impairment of glycerol metabolism. Biotechnol. Bioeng. 115 433–443. 10.1002/bit.26402 [DOI] [PubMed] [Google Scholar]
- Zhang B. X., Chen H. Q., Li M., Gu Z. N., Song Y. D., Colin R., et al. (2013). Genetic engineering of Yarrowia lipolytica for enhanced production of trans-10, cis-12 conjugated linoleic acid. Microb. Cell Fact. 12:70. 10.1186/1475-2859-12-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. X., Rong C. C., Chen H. Q., Song Y. D., Zhang H., Chen W. (2012). De novo synthesis of trans-10, cis-12 conjugated linoleic acid in oleaginous yeast Yarrowia lipolytica. Microb. Cell Fact. 11:51. 10.1186/1475-2859-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Ouellet M., Batth T. S., Adams P. D., Petzold C. J., Mukhopadhyay A., et al. (2012). Enhancing fatty acid production by the expression of the regulatory transcription factor FadR. Metab. Eng. 14 653–660. 10.1016/j.ymben.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Zhang F., Rodriguez S., Keasling J. D. (2011). Metabolic engineering of microbial pathways for advanced biofuels production. Curr. Opin. Biotechnol. 22 775–783. 10.1016/j.copbio.2011.04.024 [DOI] [PubMed] [Google Scholar]
- Zhang H., Feng Y., Cui Q., Song X. (2017). Expression of Vitreoscilla hemoglobin enhances production of arachidonic acid and lipids in Mortierella alpina. BMC Biotechnol. 17:68. 10.1186/s12896-017-0388-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang L., Chen H., Chen Y. Q., Ratledge C., Song Y., et al. (2013). Regulatory properties of malic enzyme in the oleaginous yeast, Yarrowia lipolytica, and its non-involvement in lipid accumulation. Biotechnol. Lett. 35 2091–2098. 10.1007/s10529-013-1302-7 [DOI] [PubMed] [Google Scholar]
- Zhang S., He H., Guan S., Cai B., Li Q., Rong S. (2020). Bacterial cellulose-alginate composite beads as Yarrowia lipolytica cell carriers for lactone production. Molecules 25:928. 10.3390/molecules25040928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xu Y., Jiang C. (2015). Efficient biosynthesis of γ-decalactone in ionic liquids by immobilized whole cells of Yarrowia lipolytica G3-3.21 on attapulgite. Bioprocess Biosyst. Eng. 38 2045–2052. 10.1007/s00449-015-1431-6 [DOI] [PubMed] [Google Scholar]
- Zhao Y. P., Mu X. Q., Xu Y. (2014). Improvement in γ-decalactone production by Yarrowia sp. after genome shuffling. Chem. Pap. 68 1030–1040. 10.2478/s11696-014-0551-9 [DOI] [Google Scholar]
- Zhou Y. J., Buijs N. A., Zhu Z., Qin J., Siewers V., Nielsen J. (2016). Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat. Commun. 7:11709. 10.1038/ncomms11709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Zhou Y. J., Kang M.-K., Krivoruchko A., Buijs N. A., Nielsen J. (2017). Enabling the synthesis of medium chain alkanes and 1-alkenes in yeast. Metab. Eng. 44 81–88. 10.1016/j.ymben.2017.09.007 [DOI] [PubMed] [Google Scholar]