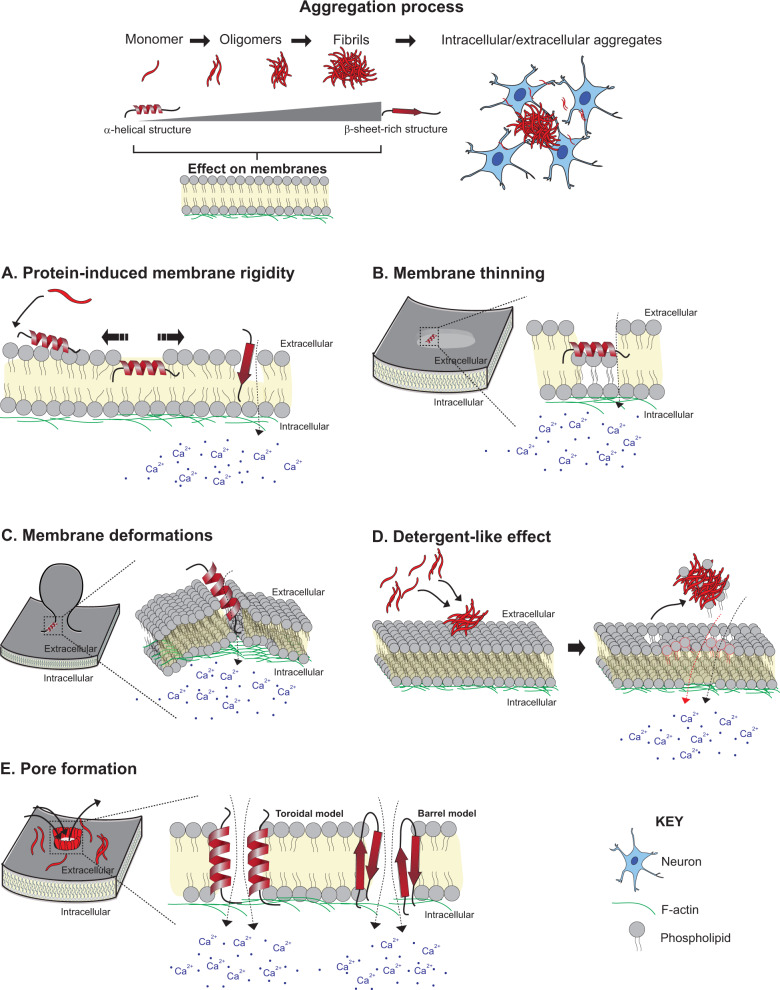
Fig. 2. Loss of membrane integrity in neurodegenerative diseases.
In Alzheimer’s disease and Parkinson’s disease, the disease proteins amyloid-β and α-synuclein, respectively, aggregate from monomers to aggregates, transitioning from intrinsically unfolded or α-helical structures to β-sheet-rich structures. Different mechanisms of protein-induced loss of membrane integrity are represented. a Protein–lipid interactions and lipid-mediated conformational changes result in protein incorporation into the membrane. This increases surface pressure and membrane rigidity, and can promote membrane thinning and deformation. b, c Proteins may induce lipids to reposition out of the plane of the membrane, resulting in membrane thinning (b) and curvature (c). d During aggregation, oligomers extract phospholipids from the bilayer and incorporate into the growing fibrils, causing membrane rupture. e Amyloidogenic proteins form pores in membranes. α-Syn forms pores rich in α-helical or β-sheet structures (toroidal and barrel models, respectively). All these mechanisms result in an influx of calcium from the extracellular environment and an efflux of cytosolic content.