Abstract
This study evaluated the pH, acidity, soluble solids, color, dietary fiber, sensory acceptance and the viability of Lactobacillus rhamnosus, Lactobacillus plantarum and Lactobacillus acidophilus in mango and carrot mixed juices. In addition, this study verified the resistance of L. plantarum that presented greater viability to the gastrointestinal tract simulated in vitro. Three formulations were elaborated (varying the pulps concentration) and the products were stored at 8 °C for 35 days. No difference was found in the total soluble solids and color of the products during storage time at 8 °C. A reduction in pH and an increase in acidity were observed in all samples during storage, probably due to the fermentative action of probiotics, which negatively influenced acceptance after 35 days of storage. On the other hand, juices with a higher concentration of mango pulp were more accepted and may be a strategy to improve the acceptance of fermented juices. Microorganisms showed greater viability in juices that had higher amount of carrot pulp, probably due to the higher fiber content in these samples. During the 35-day shelf life, all juices with L. plantarum maintained counts above 7 log CFU mL−1 after gastrointestinal conditions simulation. Therefore, mango and carrot mixed juice showed to be as a good vehicle for probiotic bacteria and meets the needs of consumers looking for functional, healthy, non-dairy and low-sugar foods.
Keywords: Mixed beverage, Vegetable, Fruit, Probiotics, In vitro gastrointestinal digestion
Introduction
Interest in the development of novel functional foods and their incorporation into a healthy diet has been growing in recent years. Among these products, those containing probiotic microorganisms have gained importance, since these bacteria promote a beneficial effect on host health and have potential for prevention of various diseases (Kandylis et al. 2016). Probiotics help maintain balance and stability of the gut microbiota, contributing to digestive functions, such as constipation control, nutritional bioavailability and modulation of digestive and immune system activity. These microorganisms have beneficial health effects related to cancer, antibiotic-associated diarrhea, traveler diarrhea, allergies, high blood pressure and cholesterol, immune system function, and vitamin production (Rostami et al. 2018).
The demand for new food matrices as a vehicle for probiotics in alternative to dairy products is increasing due to intolerance and allergy to milk derivatives from part of the population, increase of individuals who adhere to vegetarianism, cholesterol-restricted diets, among other factors (Martins et al. 2013). Thus, the use of the vegetable matrix has been widely discussed as an alternative for the incorporation of probiotic bacteria in the diet, since fruits and vegetables contain nutrients for the growth of these microorganisms without offering consumers the inconveniences related to dairy products (Martins et al. 2016; Shori 2016).
Many studies were carried out using the vegetable matrix as carrier of probiotic bacteria, such as carrot juice fermented by L. plantarum (Li et al. 2016), minimally processed melon enriched with L. acidophilus (Oliveira et al. 2017), orange juice enriched with Lactobacillus casei (Costa et al. 2017), rice extract fermented by L. plantarum (Savedboworn et al. 2017), soybean extract fermented by L. acidophilus La-5, Bifidobacterium animalis subsp. lactis Bb-12 and Streptococcus thermophilus (Battistini et al. 2018), among others. In general, the viability of probiotic bacteria in vegetable products depends on several intrinsic and extrinsic factors, such as the species and bacterial strain used, pH, the dissolved oxygen, the chemical composition of the food, as well as storage temperature, the nature of the ingredients added and the food matrix (Shori 2016).
In this context, the production of mixed juice may be a promising alternative to increase the viability of probiotic microorganisms and the sensory acceptance of the product, besides being an alternative to conventional dairy products (Antunes et al. 2013). Among the fruits and vegetables that have high production and industrial processing, mango and carrot stand out due to the low cost, high availability of nutrients and palatable flavor (Kaur and Aggarwal 2016).
Mango (Mangifera indica) is the second most important tropical fruit in the world, and is largely consumed due to its attractive color, exotic flavor, sweet taste and succulence (Guan et al. 2016). In addition, mango juice has a high nutritive value with high contents of ascorbic acid, phenols and carotenoids (Guan et al. 2016). The carrot (Daucus carota L.), besides being one of the most commonly used and well-known vegetables in the everyday kitchen (Riganakos et al. 2017), originates the carrot juice, which is considered one of the most popular vegetable juices (Li et al. 2016). Mango and carrot are vegetables rich in functional components such as carotenoids, ascorbic acid, phenolic compounds, vitamins (A, D, B, E, C and K), fiber and minerals (calcium, potassium, phosphorus, sodium and iron) (Cadena et al. 2013). Mixed juice of mango and carrot is a product with high nutritional value, because these vegetables are important dietary source of carotenoids such as alpha- and beta-carotene, lutein and lycopene. Beta-carotene, one of the most biologically active carotenoids, is a precursor of vitamin A (Riganakos et al 2017), essential for vision and growth of children. Thermal processes such as pasteurization are generally used to conserve these products, to guarantee the quality and food safety of the juices (Kaur and Aggarwal 2016). Although this technology may result in changes in sensory characteristics and reduced bioavailability of some nutrients, these losses are low and in some cases are imperceptible. Furthermore, considering microbiological safety, production scale and process cost, heat treatment is still the most advantageous and safest method applied by the food industry (Kaur and Aggarwal 2016). Therefore, the combination of mango and carrot with the incorporation of probiotic microorganisms aims to offer the consumer a nutritionally rich product with great sales potential.
However, no study evaluated the development of the probiotic microorganisms in mixed juice of mango and carrot, as well as the characteristics of the product and the impact of the different concentrations of each vegetable. Thus, this study evaluated the viability of probiotic bacteria (L. rhamnosus GG, L. plantarum LP299V® and L. acidophilus LA-14) and the physicochemical and sensory characteristics of different formulations of mango and carrot mixed juice during 35 days at 8 °C. In addition, the resistance of the probiotic strain possessing the greater viability in the different formulations of mixed juice to in vitro simulated gastrointestinal conditions was evaluated.
Material and methods
Preparation of mango and carrot pulps
The mango pulp (Mangifera indica L. Ubá variety) was obtained from the company Bela Ischia Ltda (Astolfo Dutra, MG, Brazil) and kept frozen at − 18 ºC until preparation of mixed juices.
For obtaining carrot pulp (Daucus carota L.), fresh roots were obtained from the local market of the city of Rio Pomba (Minas Gerais, Brasil), washed in running water and sanitized in organic chlorine solution at 200 mg L-1 of active chlorine for 15 min at 5 ºC. After the sanitization, the vegetables were rinsed in chlorinated solution at 20 mg L-1 for 5 min and then peeled and chopped with the aid of knives. The carrot pulp was obtained by milling the roots with water, in the proportion of 1:1, in a domestic blender. Immediately after obtaining the carrot pulp, the mixed juice was formulated as described in Sect. 2.2.
Preparation and processing of mango and carrot mixed juices
To prepare the formulations, three different formulations of mango and carrot mixed juice were tested: formulation 1 (F1): 5 parts mango pulp and 1 part carrot pulp; formulation 2 (F2): 2 parts mango pulp and 1 part carrot pulp; and formulation 3 (F3): 1 part mango pulp and 1 part carrot pulp. The proportions of vegetable pulps were defined as equidistant concentrations, in which the amount of carrot never exceeded the mango quantity due to the mango being highly appreciated by the consumers, for its pleasant taste and high production. After mixing the pulps, mineral water was added until the final concentration of the pulps reached 60% (w/v). Subsequently, the total soluble solids (TSS) was adjusted to 8°Brix with the addition of commercial sucrose in all formulations to standardize the content of soluble solids in mixed juices. The sugar content added to the formulations was approximately 1.5%. These values were selected according to Brazilian legislation to classify it as ready-to-drink sweetened tropical juice (Brazil 2018).
Aliquots of 100 mL of the mixed juices were filled into sterilized glass bottles with metal caps and pasteurized at 90 ºC/1 min for further analysis (Faraoni et al. 2013). This pasteurization binomial was chosen based on food safety and minimal impacts on the composition of juices (Faraoni et al. 2013; Moreira et al. 2017). Pasteurization was carried out in a water bath and a thermocouple was added in the lower heating point on a control bottle containing juice to monitor the temperature. After pasteurization, the product was cooled to 10 ºC with ice water and stored at 8 ºC (Moreira et al. 2017).
Preparation and addition of probiotics in mixed juice
In this study, the probiotic cultures of L. rhamnosus GG (Culturelle®, lyophilized capsules) (LGG), L. plantarum LP299V® (JamiesonTM Natural Sources, lyophilized capsules) (LP) and L. acidophilus LA-14 (Nature's Bounty®, lyophilized capsules) (LA) were used. The preparation of probiotic cultures and its addition to the mixed juice was carried out as described by Moreira et al. (2017), where, the pre-inoculum was prepared for each microorganism (LGG, LP and LA) in each formulation (F1, F2, and F3), totaling 9 treatments (F1LGG; F2LGG; F3LGG; F1LP; F2LP; F3LP; F1LA; F2LA; F3LA). For this, after pasteurization, one L. rhamnosus and one L. plantarum capsule, containing 1010 cells each, and five L. acidophilus capsules, containing 2 × 109 cells each (totaling ~ 1010 cells), were added aseptically and individually to 100 mL of each juice formulation. Then, the juices were manually stirred and incubated at 37 °C/24 h (Moreira et al. 2017). This product was called the pre-inoculum (PI). After 24 h, 10 mL of PI was added to 90 mL of pasteurized mixed juice in sterile glass bottles with metal caps (Moreira et al. 2017). The products obtained were stored at 8 °C for 35 days.
As control treatments, the three formulations of mango and carrot mixed juice (F1, F2, and F3) were obtained without the addition of probiotic cultures and samples were stored for 35 days at 8 °C. This binomial of storage time and temperature was chosen based on the conditions commonly used for commercialization and consumption of pasteurized juice (Oliveira et al. 2017).
pH, titratable acidity, total soluble solids and color of the mango and carrot mixed juices
Mango and carrot mixed juices were evaluated at times 0, 7, 14, 21, 28 and 35 days of storage at 8 °C. Analysis of pH, titratable acidity and TSS of mixed juices were carried out according to the AOAC methods: 920.92, 10.197 e 973.21, respectively (AOAC 2000). For this, 10 g of each sample was weighed and 100 mL of distilled water added. The pH was determined by direct reading in potentiometer (mPA-210, MS TECNOPON, São Paulo, Brazil). For acidity determination, expressed as a percentage of citric acid (w/v), the juice was titrated with 0.1 mol L−1 NaOH standard solution until a final pH of 8.2–8.4 (titration endpoint) was reached. The TSS was performed with a direct reading on refractometer (Digital ABBE Refractometer WAY-2S, Quimis, Brazil) at 25 °C, and the results were expressed in ºBrix.
The color of the mixed juices was measured using a Color Reader CR-10 (Minolta) colorimeter. The color measurements were performed by direct reading of reflectance of the L*, a* and b* coordinates using the CIELAB scale. For each sample, three readings were taken at different random points of the product in order to obtain the mean result. The total color difference (ΔE*) between the samples was measured according to Eq. 1 (Adekunte et al. 2010). In each formulation, the mixed juice without addition of probiotic was arbitrarily defined as the reference.
| 1 |
Sensory analysis
Sensory acceptance of the mixed juices after processing (zero day) and after 35 days of storage at 8 ºC were carried out by 118 non-trained panelists, in individual booths under white light. A volume of 20 mL (8 ± 2 °C) of each sample was monadically served in plastic cups coded with three-digit random numbers. A balanced presentation order was used to minimize the effects of the first order and carry over effects. The panelists evaluated the samples using a nine point structured hedonic scale varying from “disliked extremely” (1) to “liked extremely” (9) for the global impression (Stone and Sidel 1985). The sensory evaluation was approved by the Ethics in Research Commission of the Federal University of Viçosa (Protocol 2.020.120).
Total dietary fiber content
The total dietary fiber content was carried out by the enzymatic–gravimetric method according to AOAC (2012) for the three formulations (F1, F2 and F3) after processing. This method is based on sequential enzymatic digestion of the samples by α-amylase, protease and amyloglucosidase for hydrolysis of starch, proteins and amylose, respectively, followed by ethanol precipitation to obtain the soluble fiber and subsequent drying of the residue to obtain the insoluble fiber. The total dietary fiber content is the sum of the soluble and insoluble fractions of the product.
Probiotic cultures viability in mango and carrot mixed juice
Aliquots of 25 mL of the mixed juices were homogenized in 225 mL of saline solution, obtaining the dilution 10–1. Subsequently, serial dilutions were performed. The viability of L. rhamnosus, L. plantarum and L. acidophilus was carried out after 0, 7, 14, 21, 28 and 35 days of mixed juices processing stored at 8 ºC, by colony counting using pour plate method in MRS (Kasvi, Brazil) culture medium (Richter and Vedamuthu 2001). The plates were incubated at 37 ºC for 72 h. The control samples, not added to the probiotic microorganisms, were also submitted to the same test conditions. The results were expressed in log CFU mL−1 of the product.
Evaluation of the survival of the probiotic microorganism to in vitro simulated gastrointestinal conditions
For the in vitro assay, the microorganism that presented the highest viability was chosen and the 3 formulations (F1, F2 and F3) were evaluated for this microorganism, in order to evaluate the resistance of this probiotic to the gastrointestinal conditions and if the medium composition influenced their survival. The analysis was carried out after 0 and 35 days of the mixed juices processed and stored at 8 ºC.
The analysis was carried out using an in vitro model, through the simulation of gastric and enteric juices and enzymes of the gastrointestinal tract according to methodology proposed by Bedani et al. (2013). Diluted aliquots of probiotic mixed juices were transferred to sterile glass vials and the pH was adjusted to 2.3–2.4 with 1 mol L−1 HCl. Pepsin (from porcine stomach mucosa, P7000, Sigma–Aldrich) and lipase (Amano lipase G, from Penicilium camemberti, Sigma–Aldrich) solutions were added to samples to reach a concentration of 3 g L−1 and 0.9 mg L−1, respectively. Flasks were incubated at 37 °C, with agitation during 2 h, leading to the simulated gastric phase. Then, in enteral phase I, the pH was increased to 5.4–5.7 with alkaline solution [150 mL of 1 mol L−1 NaOH (Synth, Diadema, Brazil) and 14 g of PO4H2Na.2H2O (Synth, Diadema, Brazil) and distilled water up to 1 L] containing bovine bile (B3883, Sigma-Aldrich) and pancreatin from porcine pancreas (P7545, Sigma-Aldrich) at a concentration of 10 g L−1 and 1 g L−1, respectively. The samples were incubated again at 37 °C for 2 h under agitation. Finally, the simulation of the enteric phase II occurred. The pH was increased to 6.8–7.2 using the same alkaline solution, bile and pancreatin were adjusted to maintain the concentration of 10 g L−1 and 1 g L−1, respectively, and the samples were incubated again at 37 °C for 2 h under agitation, and reaching 6 h of assay.
The enumeration of the probiotic microorganisms surviving the gastrointestinal conditions was carried out after the end of each phase (2 h = gastric phase (GP), 4 h = enteric phase I (EP I) and 6 h = enteric phase II (EP II)) and viability was carried out according to Sect. 2.5.
Statistical analysis
Each sample treatment was carried out in triplicate with three determinations per analysis. Data was analyzed via Analysis of variance (ANOVA) using the STATISTICA 7.0 software (StatiSoft, Inc., Tulsa, Okla., USA). Tukey test was used to determine the differences among the treatment means at the 95% confidence level when appropriate. Data was represented as the mean ± standard deviation.
Results and discussion
pH, acidity and viability of probiotics in the pre-inoculum of mango and carrot mixed juices after 24 h of incubation at 37 ºC
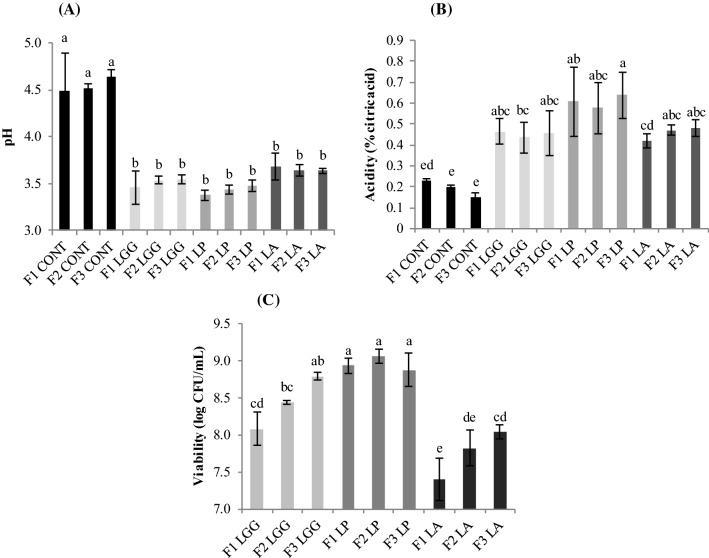
The pre-inoculum of mango and carrot mixed juices after 24 h of incubation at 37 ºC showed lower pH and higher acidity compared to control juices (without addition of probiotic cultures) (p < 0.05—Fig. 1a, b). Similar counts of probiotic cultures were inoculated at pre-inoculum and L. plantarum was the microorganism that showed the highest viability after 24 h of incubation, independent of the formulation, being similar to L. rhamnosus in formulation 3 (Fig. 1c).
Fig. 1.
Evaluation of pH (a), acidity (b) and viability of probiotic microorganisms (c) in pre-inoculum after 24 h of incubation at 37 ºC. F1: 5 parts mango pulp/1 part of carrot pulp. F2: 2 parts mango pulp/1 part of carrot pulp. F3: 1 part mango pulp/1 part of carrot pulp. CONT: without addition of probiotic microorganism. LGG: L. rhamnosus GG (LGG). LP: L. plantarum LP299V®. LA: L. acidophilus LA-14. Different letters indicate significant difference (p < 0.05) among the treatments
L. plantarum has a good adaptability to different media being found in several ecological niches, as well as in the gastrointestinal tracts of humans and animals (Sabo et al. 2014). This good adaptability is associated with its high capacity to ferment a variety of sugars, as well as the rapid absorption capacity of solutes due to the high efficiency in the transport system (Salminen and Von Wright 1993).
The results show that there is a difference during the pre-inoculation fermentation in relation to the adaptation of probiotic cultures to the juice formulations, being L. acidophilus the bacterium of lower robustness. Other studies corroborate the influence of the medium composition on the viability of different probiotic lactobacilli cultures (Espirito-Santo et al. 2015). These authors report that a possible explanation for the better lactobacilli viability could be the gradual adaptation of the cultures to the stressful conditions of the fruit juices (Espirito-Santo et al. 2015).
According to Sheehan et al. (2007), changes in pH and acidity may be useful to indicate the adaptation and growth of bacteria in different fruit juices. However, other factors should also be considered such as the chemical composition of juices, the balance between nutrients, inhibitory compounds, intrinsic factors and the ability of the strains to adapt to these matrices. Thus, L. acidophilus showed lower development compared to other microorganisms in the formulations prepared in this study, possibly because the environment conditions were not favorable to the development of this fastidious microorganism, such as pH, oxygen level and nutrient availability.
Physicochemical characteristics of mango and carrot probiotic mixed juice
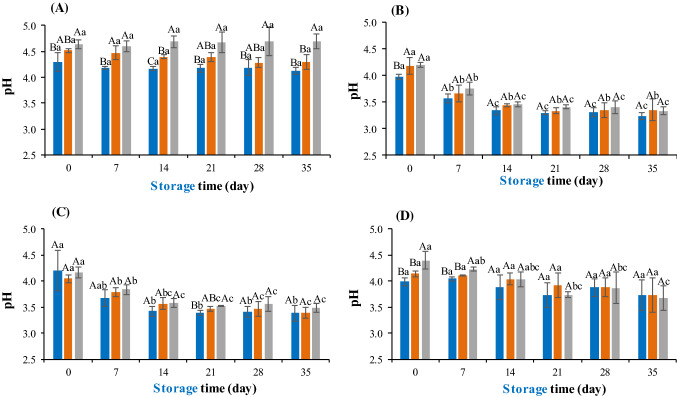
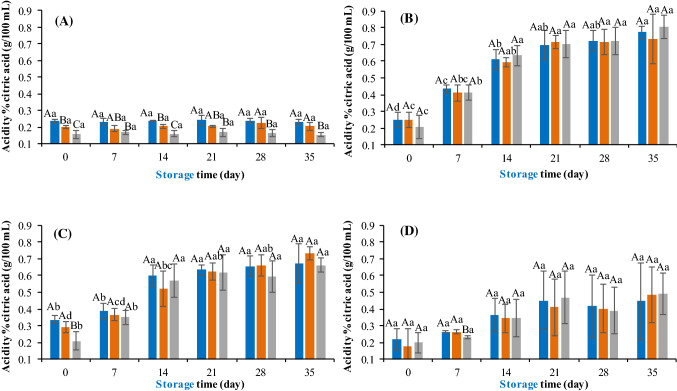
Figures 2 and 3 show the results obtained for the pH and acidity, respectively, of the mixed juices in the different formulations, added with the respective pre-inoculum containing the probiotic microorganisms.
Fig. 2.
Evaluation of the pH of different formulations of probiotic mixed juices. a Control. b Juice containing L. rhamnosus. c Juice containing L. plantarum. D: Juice containing L. acidophilus. F1 (blue bar), F2 (orange bar), F3 (gray bar). Different upper case letters indicate significant difference (p < 0.05) among the formulations at the same time and different lowercase letters indicate significant difference (p < 0.05) over time for each formulation
Fig. 3.
Evaluation of the acidity of the different formulations of probiotic mixed juices. a Control. b Juice containing L. rhamnosus. c Juice containing L. plantarum. d Juice containing L. acidophilus. F1 (blue bar), F2 (orange bar), F3 (gray bar). Different upper case letters indicate significant difference (p < 0.05) among the formulations at the same time and different lowercase letters indicate significant difference (p < 0.05) over time for each formulation
In the comparative evaluation of the formulations without addition of probiotics, it was verified that formulation 3 had a higher pH value (Fig. 2a) and a lower acidity value (Fig. 3a) (p < 0.05), which is due to the increase in the amount of carrot pulp (pH 5.0) and to the decrease in the amount of mango pulp (pH 3.8). However, for the juice added to the probiotic microorganisms, independently of the added microorganism, this difference was minimized for most of the evaluated days (p > 0.05) (Figs. 2b–d, 3b–d).
Over time, the juices from the control treatments showed no change in pH and acidity (p > 0.05) (Figs. 2 and 3a). This is due to the effectiveness of thermal treatment in microbial inactivation and application of good manufacturing practices during processing and storage. On the other hand, the juices containing probiotics, with the exception of formulations 1 and 2 added by L. acidophilus, showed a reduction in pH throughout storage at 8 °C (p < 0.05) (Fig. 2b–d). Similarly, an increase in acidity was observed in these juices, except for formulations containing L. acidophilus, during 35 days of storage at 8 °C (p < 0.05) (Fig. 3b–d).
The pH reduction and acidity increase in juices were expected due to the production of organic acids as lactic and acetic acid by the added microorganisms (especially L. plantarum and L. rhamnosus—Ceapa et al. 2016; Sabo et al. 2014) during refrigerated storage where L. plantarum and L. acidophilus used sucrose, fructose and glucose as substrate present in juices (Gilliland and Speck 1997; Hedberg et al. 2008), and L. rhamnosus used glucose and fructose (Hedberg et al. 2008). In contrast, L. acidophilus, which finished the pre-inoculation phase with lower viability, showed low adaptation in these matrices, justifying the slight change in the characteristics of the juice containing this bacterium.
Pimentel et al. (2015) verified that the addition of Lactobacillus paracasei ssp. paracasei caused a slight increase in acidity as well as decreased pH levels in clarified apple juice during 28 days at 4 °C. These researchers reported that probiotic microorganisms may metabolize the simple sugars present in the juice and, consequently, produce small amounts of organic acids; or still, dead bacteria may be able to release enzymes that might have hydrolyzed the polysaccharides from the juice (Ding and Shah 2008).
There was no difference in total soluble solids (TSS) among the control samples and the samples containing probiotic microorganisms in each time evaluated (p > 0.05) and throughout the storage period (p > 0.05) (data not shown). After processing, the formulations 1, 2 and 3 showed an average of 7.9 ± 0.4, 7.9 ± 0.3, and 7.6 ± 0.4 ºBrix, respectively (p > 0.05). After 35 days of storage, these values were 7.4 ± 0.3, 7.5 ± 0.5, and 7.3 ± 0.6 ºBrix, respectively (p > 0.05). A possible reason for the maintenance of the TSS content could be due to the action of microorganisms in the hydrolysis of insoluble sugars, promoting a balance of TSS content during storage (Oliveira et al 2017).
Color
No difference was observed in L*, a* and b* parameters among the evaluated samples after the processing as well as during the storage period (p > 0.05). In general, formulations 1, 2 and 3 not added to probiotic microorganisms showed L* values of 37.2 ± 2.1; 37.2 ± 1.8 and 36.7 ± 1.6, respectively; a* values of 9.6 ± 3.3; 12.2 ± 4.4 and 13.1 ± 4.3, respectively; and, finally, b* values of 19.6 ± 7.8; 19.7 ± 7.3 and 18.0 ± 7.1, respectively. In addition, the presence of probiotic microorganisms did not affect the L*, a* and b* values of the different formulations (p > 0.05) in relation to the respective control formulation. Based on the a* and b* parameters, the samples were characterized by the coloration ranging from reddish orange to purple red. This coloration is justified by the presence of carotenoids, which is the main pigment present in the mango and carrot pulps.
Regarding the total color difference (ΔE*), arbitrarily considering each formulation of the mixed juice without addition of probiotic as reference, it was verified that the juices containing L. rhamnosus, L. plantarum and L. acidophilus presented ΔE* average of 0.9 ± 0.5; 1.3 ± 1.0 and 1.4 ± 0.6, respectively. The ΔE* represents the visual perception of the human eye and considers the color changes perceived by consumers as: very visible change (ΔE* > 3.0), notable (1.5 < ΔE* < 3.0) and no visible color change (ΔE* < 1.5) (Adekunte et al. 2010). According to Choi et al. (2002), only ΔE* values greater than 2 correspond to visually perceptible color changes between two samples. Thus, regardless of the formulation, it is expected that the addition of probiotic microorganisms will not result in perceptible color change by the consumer.
Sensory analysis of mango and carrot probiotic mixed juice
Table 1 shows the results obtained in the sensory analysis of the mixed juices. In the general evaluation, the juices were well evaluated by panelists for overall impression both after processing and after 35 days of storage (score > 6.0). In detail, the addition of the probiotic cultures did not affect the acceptance of samples after processing (p > 0.05). On the other hand, after 35 days of storage, the juices without probiotics presented the highest averages compared to probiotic juices (p < 0.05). This demonstrates that the fermentative action of probiotic microorganisms in the juice during the shelf life of the product negatively affected the acceptance, probably due to the higher acidity of the probiotic juices.
Table 1.
Sensory acceptance of the different formulations of probiotic mixed juices
| Formulation type | Global impression | |
|---|---|---|
| 0 days stored- sample | 35 days stored- sample | |
| F1 without probiotic | 8.6 ± 0.4a | 8.2 ± 0.4a |
| F2 without probiotic | 8.4 ± 0.5a | 8.0 ± 0.2a |
| F3 without probiotic | 7.2 ± 0.3b | 7.3 ± 0.3b |
| F1 with L. rhamnosus | 8.4 ± 0.3a | 7.3 ± 0.3b |
| F2 with L. rhamnosus | 8.2 ± 0.4a | 6.4 ± 0.3d |
| F3 with L. rhamnosus | 7.1 ± 0.2b | 6.0 ± 0.4d |
| F1 with L. plantarum | 8.5 ± 0.4a | 7.5 ± 0.2b |
| F2 with L. plantarum | 8.3 ± 0.4a | 6.5 ± 0.3cd |
| F3 with L. plantarum | 7.3 ± 0.2b | 6.2 ± 0.4d |
| F1 with L. acidophilus | 8.4 ± 0.3a | 7.1 ± 0.3bc |
| F2 with L. acidophilus | 8.2 ± 0.5a | 6.4 ± 0.4cd |
| F3 with L. acidophilus | 7.3 ± 0.4b | 6.2 ± 0.2d |
F1: 5 parts mango pulp/1 part of carrot pulp. F2: 2 parts mango pulp/1 part of carrot pulp. F3: 1 part mango pulp/1 part of carrot pulp. Different letters in column indicate significant difference (p < 0.05) among the treatments in each time
Regarding the formulations with different concentrations of pulps, it was found that the samples that contained a higher concentration of mango pulp showed greater acceptance both after processing and after 35 days of storage (p < 0.05). Therefore, it is noteworthy that the mango pulp has great sensory appeal and can be used as a strategy to improve the acceptance of probiotic juices during storage time.
Viability of probiotic microorganisms and total dietary fiber content in mango and carrot mixed juices
The control samples (without addition of probiotic microorganisms) showed counts < 1 log CFU mL−1 for lactic acid bacteria after processing and during storage for 35 days at 8 °C (data not shown). This result proves that the viability found in juices is due only to the survival of the probiotic microorganisms added, in addition, it shows that the thermal treatment employed was efficient and that there was no subsequent contamination of the product.
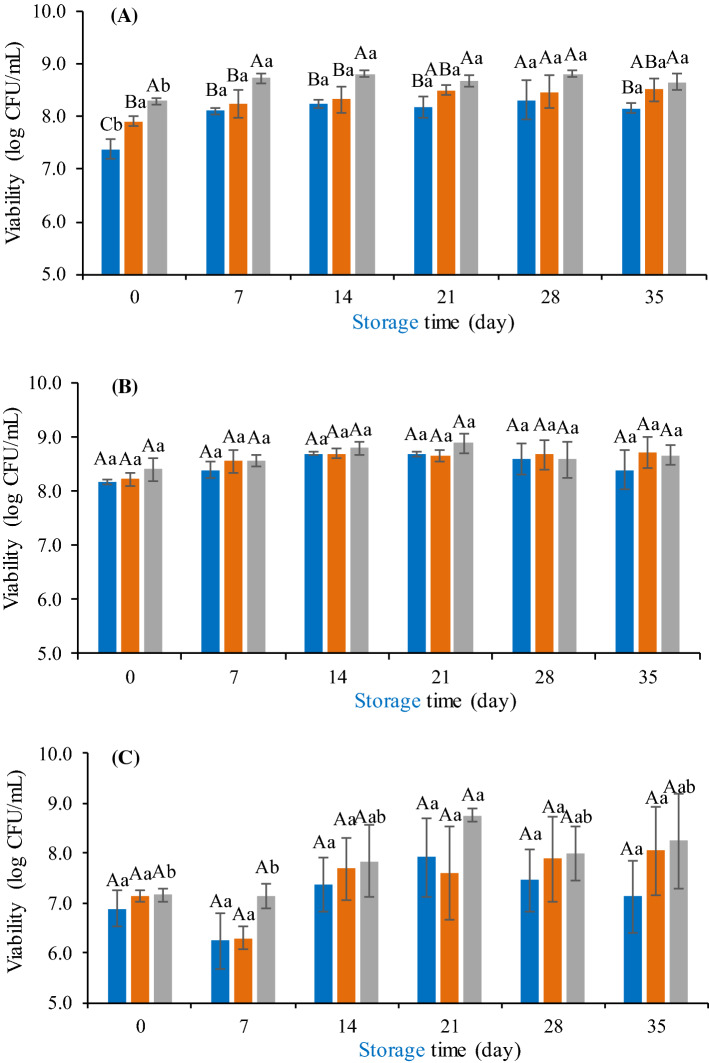
Regardless of the microorganism evaluated, formulation 3 promoted greater viability of the added microorganisms, however, only for L. rhamnosus GG this difference was statistically significant (p < 0.05) (Fig. 4a). This result is possibly due to the higher dietary fiber content present in the formulation 3 (8.9 g·100 g−1) compared to the other formulations (7.1 g·100 g−1 in the formulation 1 and 7.8 g·100 g−1 in the formulation 2) (p < 0.05).
Fig. 4.
Evaluation of the probiotic microorganisms viability in the different formulations of mixed juices. a Juice containing L. rhamnosus. b Juice containing L. plantarum. c Juice containing L. acidophilus. F1 (blue bar), F2 (orange bar), F3 (gray bar). Different upper case letters indicate significant difference (p < 0.05) among the formulations at the same time and different lowercase letters indicate significant difference (p < 0.05) over time for each formulation
According to Nualkaekul et al. (2011), dietary fibers significantly influence the cellular survival of microorganisms after processing and during the shelf life of the product. Soluble fibers can be used as substrates for the growth of probiotic microorganisms and insoluble fibers may have a protective effect on these bacteria by promoting a physical barrier during passage through the gastrointestinal tract (Ray and Montet 2014). In addition, the chemical composition of juices, pH and organic acid concentration may also directly influence the viability of probiotic microorganisms. In this context, formulation 3 presented higher pH and lower acidity, contributing to a lower inhibition of the microorganisms present in these samples compared to the other formulations.
No reduction in the count of evaluated microorganisms was observed during the storage period independent of the formulation (p > 0.05) (Fig. 4), which demonstrates the efficiency of the use of vegetable matrices, such as mango and carrot, as a source for the growth and maintenance of probiotic bacteria.
The highest average counts were obtained for L. plantarum (8.56 ± 0.19 log CFU mL−1) and L. rhamnosus (8.35 ± 0.35 log CFU mL−1) and L. acidophilus showed lower viability (7.49 ± 0.65 log CFU mL−1) after 35 days of storage at 8 °C (p < 0.05). The same behavior was observed in the pre-inoculum of the mixed juice after 24 h of incubation at 37° C. Therefore, from the results obtained, it was verified that L. plantarum showed better adaptation in the juice prepared with the matrices tested, which corroborates with Kleerebezem et al. (2003), who reported that L. plantarum has a high proportion of resistance genes, which play an important role in the interaction of the bacterium with the environment, resulting in a better viability compared to the other evaluated microorganisms. On the other hand, L. acidophilus showed a slower metabolism, which is confirmed by the lower counts, in absolute terms, in the more acidic formulations (formulations 1 and 2) (Fig. 4).
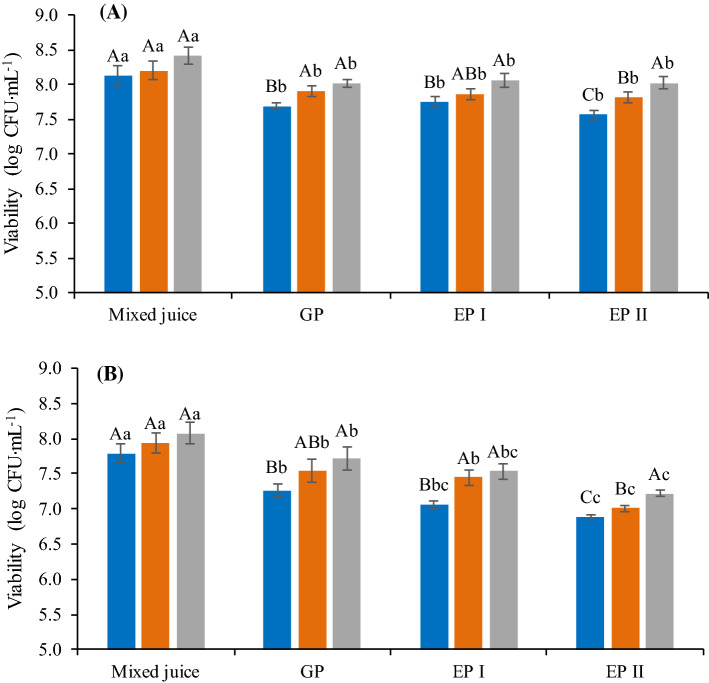
Survival of L. plantarum to in vitro simulated gastrointestinal conditions
To exert beneficial effects on host health, probiotic microorganisms must resist the gastrointestinal conditions and to assess cell viability in the digestion phases, gastrointestinal conditions were simulated by in vitro assay. In order to carry out the study, L. plantarum was chosen because it presented the best viability, in absolute terms, in the mixed juice, regardless of the formulation, during refrigerated storage.
The viability of L. plantarum in the mixed juice pasteurized and stored for 35 days at 8 °C was decreased in the gastric phase (GP) (p < 0.05) (Fig. 5). This is probably due to the low pH of this phase (pH value of 2.5), which may have caused a partially lethal stress to the L. plantarum cells. In enteric phases I and II (EP I and II), the viability of L. plantarum remained similar to GP for the 0 day—stored sample (p > 0.05) (Fig. 5a), but for 35 days—stored sample, there was a reduction in the count after EP II compared to GP (p < 0.05) (Fig. 5b).
Fig. 5.
Survival of L. plantarum in different formulations of mango and carrot mixed juice submitted to in vitro simulated gastrointestinal conditions. Mixed juice: Mango and carrot mixed juice prior to initiation of in vitro simulation; GP: Gastric phase; EP I: Enteric phase I; EP II: Enteral phase II; a Evaluation after processing (0 day). b Evaluation after 35 days of mixed juice storage at 8 °C. F1 (blue bar), F2 (orange bar), F3 (gray bar). Different upper case letters indicate significant difference (p < 0.05) among the formulations in the same phase and different lowercase letters indicate significant difference (p < 0.05) among the phases for the same formulation
The enteric phases I and II, which simulate the small and large intestine, respectively, present higher pH, being 5.4–5.7 for EP I and 6.8–7.2 for EP II. These higher pH values may have led to maintenance of probiotic viability, ensuring that the microorganism reaches the intestine with counts above 6 log CFU mL−1, colonizing it and exerting positive effects on the host related to antibiotic-associated diarrhea, vitamin production, improvement of immune system function and control of high blood pressure and cholesterol (Rostami et al. 2018).
Formulation 3 showed the highest counts after passage through the simulated gastrointestinal conditions for both evaluated times (p < 0.05) (Fig. 5), confirming the best results found for this formulation in the evaluation of viability in the mixed juice. Therefore, it was evident that a higher amount of carrot in the juice compared to the other formulations, promoted better conditions for the maintenance of viability of L. plantarum, contrary to formulation 1 that promoted less viability in general terms.
From these results, it can be verified that the different formulations affected the viability and survival to gastrointestinal tract of the probiotic microorganisms, in which the best intrinsic conditions (pH, fermentable sugars availability) and the higher fiber content (resulting in a more structured matrix) of the formulation 3 favored a better survival of microorganisms.
All formulations maintained counts above 7 log CFU mL−1 at the end of EP II after 35 days of storage at 8 °C (Fig. 5), confirming that mixed juice of mango and carrot is a great vehicle for probiotic bacteria. This result suggests that different vegetable matrices can serve as substrate for the growth and maintenance of probiotic microorganisms, resulting in a high number of viable cells.
In order to exert beneficial effects on the host, it is necessary to ingest at least 6 to 7 log CFU of viable cells of probiotic microorganisms daily (Martins et al. 2016). Based on this, in order to have a probiotic effect of the mango and carrot mixed juice added of L. plantarum, a daily portion of 100 mL of the product is sufficient for ingestion of more than 9 log CFU mL−1 viable cells.
Conclusion
A reduction in pH and an increase in acidity were observed in all probiotic mixed juices during storage, probably due to the fermentative action of these microorganisms. On the other hand, no difference was found in the total soluble solids and color of the products. In addition, mixed juice of mango and carrot can be considered a great vehicle for probiotic bacteria. Formulations that contained higher concentration of carrot pulp in composition promoted better the viability of probiotic cultures, probably due to higher concentration of fibers. On the other hand, the higher concentration of mango pulp increased the sensory acceptance of the juices. L. plantarum was highlighted with better viability and survived to in vitro simulated gastrointestinal conditions. At the end of this simulation, this microorganism presented counts higher than 7 log CFU mL−1, which is enough for this bacterium to exert its probiotic effect. Our results show that mango and carrot mixed juice may be an alternative for the incorporation of these microorganisms in the diet of individuals who cannot consume dairy products, as well as other nutrients from mango and carrot such as vitamins, minerals, antioxidants and fibers, which give healthier characteristics to the product.
Acknowledgments
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adekunte AO, Tiwari BK, Cullen PJ, et al. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010;122:500–507. doi: 10.1016/j.foodchem.2010.01.026. [DOI] [Google Scholar]
- Antunes AEC, Liserre AM, Coelho ALA, et al. Acerola nectar with added microencapsulated probiotic. LWT: Food Sci Technol. 2013;54:125–131. doi: 10.1016/j.lwt.2013.04.018. [DOI] [Google Scholar]
- AOAC (2000) Official methods of analysis of the AOAC, 16 edn. Washington, DC
- AOAC . Official methods of analysis of AOAC International: Agricultural chemicals, contaminants, drugs. 16. Gaithersburg: AOAC International; 2012. [Google Scholar]
- Battistini C, Gullón B, Ichimura ES, Gomes AMP, Ribeiro EP, Kunigk L, Moreira JUV, Jurkiewicz C. Development and characterization of an innovative synbiotic fermented beverage based on vegetable soybean. Braz J Microbiol. 2018;49(2):303–309. doi: 10.1016/j.bjm.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedani R, Rossi EA, Saad SMI. Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol. 2013;34:382–389. doi: 10.1016/j.fm.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Brazil (2018) Ministério da Agricultura, Pecuária e Abastecimento. Instrução normativa nº 49, de 26 de Setembro de 2018. Regulamenta os padrões de identidade e qualidade de suco e polpa de fruta (Brasília, DF), Diário Oficial da União.
- Cadena RS, Cruz AG, Netto RR, et al. Sensory profile and physicochemical characteristics of mango nectar sweetened with high intensity sweeteners throughout storage time. Food Res Int. 2013;54:1670–1679. doi: 10.1016/j.foodres.2013.10.012. [DOI] [Google Scholar]
- Ceapa C, Davids M, Ritari J, et al. The variable regions of Lactobacillus rhamnosus genomes reveal the dynamic evolution of metabolic and host-adaptation repertoires. Genome Biol Evol. 2016;8:1889–1905. doi: 10.1093/gbe/evw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MH, Kim GH, Lee HS. Effects of ascorbic acid retention on juice color and pigment stability in blood Orange (Citrus sinensis) juice during refrigerated storage. Food Res Int. 2002;35:753–759. doi: 10.1016/S0963-9969(02)00071-6. [DOI] [Google Scholar]
- Costa GM, Silva JVC, Mingotti JD, et al. Effect of ascorbic acid or oligofructose supplementation on L. paracasei viability, physicochemical characteristics and acceptance of probiotic orange juice. LWT: Food Sci Technol. 2017;75:195–201. doi: 10.1016/j.lwt.2016.08.051. [DOI] [Google Scholar]
- Ding WK, Shah NP. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int Food Res J. 2008;15:219–232. [Google Scholar]
- Espirito-Santo AP, Carlin F, Renard CMGC. Apple, grape or orange juice: Which one offers the best substrate for lactobacilli growth? A screening study on bacteria viability, superoxide dismutase activity, folates production and hedonic characteristics. Food Res Int. 2015;78:352–360. doi: 10.1016/j.foodres.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Faraoni AS, Ramos AM, Guedes DB, Pinto MRMR. Propriedades reológicas de sucos mistos de manga, goiaba e acerola adicionados de fitoquímicos. Braz J Food Technol. 2013;16:21–28. doi: 10.1590/S1981-67232013005000002. [DOI] [Google Scholar]
- Gilliland SE, Speck ML. Use of the Minitek System for characterizing Lactobacilli. Appl Environ Microbiol. 1997;33:1289–1292. doi: 10.1128/AEM.33.6.1289-1292.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Zhou L, Bi J, et al. Change of microbial and quality attributes of mango juice treated by high pressure homogenization combined with moderate inlet temperatures during storage. Innov Food Sci Emerg Technol. 2016;36:320–329. doi: 10.1016/j.ifset.2016.07.009. [DOI] [Google Scholar]
- Hedberg M, Hasslöf P, Sjöström I, et al. Sugar fermentation in probiotic bacteria: an in vitro study. Oral Microbiol Immunol. 2008;23:482–485. doi: 10.1111/j.1399-302X.2008.00457.x. [DOI] [PubMed] [Google Scholar]
- Kandylis P, Pissaridi K, Bekatorou A, et al. Dairy and non-dairy probiotic beverages. Curr Opin Food Sci. 2016;7:58–63. doi: 10.1016/j.cofs.2015.11.012. [DOI] [Google Scholar]
- Kaur G, Aggarwal P. Effect of thermal processing and chemical preservatives on the physicochemical and phytochemical parameters of carrot juice. Asian J Dairy Food Res. 2016;35:71–75. [Google Scholar]
- Kleerebezem M, Boekhorst J, Van Kranenburg R, et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci. 2003;100:1990–1995. doi: 10.1073/pnas.0337704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Nie SP, Zhu KX, et al. Lactobacillus plantarum NCU116 fermented carrot juice evokes changes of metabolites in serum from type 2 diabetic rats. Food Res Int. 2016;80:36–40. doi: 10.1016/j.foodres.2015.12.025. [DOI] [Google Scholar]
- Martins EMF, Ramos AM, Vanzela ESL, et al. Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res Int. 2013;51:764–770. doi: 10.1016/j.foodres.2013.01.047. [DOI] [Google Scholar]
- Martins EMF, Ramos AM, Martins ML, Leite Junior BRC. Fruit salad as a new vehicle for probiotic bacteria. Food Sci Technol. 2016;36:540–548. doi: 10.1590/1678-457X.03316. [DOI] [Google Scholar]
- Moreira RM, Martins ML, Leite Júnior BRC, et al. Development of a juçara and Ubá mango juice mixture with added Lactobacillus rhamnosus GG processed by high pressure. LWT - Food Sci Technol. 2017;77:259–268. doi: 10.1016/j.lwt.2016.11.049. [DOI] [Google Scholar]
- Nualkaekul S, Salmeron I, Charalampopoulos D. Investigation of the factors influencing the survival of Bifidobacterium longum in model acidic solutions and fruit juices. Food Chem. 2011;129:1037–1044. doi: 10.1016/j.foodchem.2011.05.071. [DOI] [PubMed] [Google Scholar]
- Oliveira PM, Ramos AM, Martins EMF, et al. Comparison of vacuum impregnation and soaking techniques for addition of the probiotic Lactobacillus acidophilus to minimally processed melon. Int J Food Sci Technol. 2017;52:2547–2554. doi: 10.1111/ijfs.13540. [DOI] [Google Scholar]
- Pimentel TC, Madrona GS, Garcia S, Prudencio SH. Probiotic viability, physicochemical characteristics and acceptability during refrigerated storage of clarified apple juice supplemented with Lactobacillus paracasei ssp. paracasei and oligofructose in different package type. LWT: Food Sci Technol. 2015;63:415–422. doi: 10.1016/j.lwt.2015.03.009. [DOI] [Google Scholar]
- Ray RC, Montet D (2014) Microorganisms and fermentation of traditional foods. In: Kohajdová Z (ed.) Fermented cereal products. CRC Press, Boca Raton
- Richter RL, Vedamuthu ER (2001) Milk and milk products. In: Downes FP, & Ito K (eds.) Compendium of methods for the microbiological examination of foods, 4 edn. Washington: American Public Health Association—APHA, pp 483–495.
- Riganakos KA, Karabagias IK, Gertzou I, Stahl M. Comparison of UV-C and thermal treatments for the preservation of carrot juice. Innov Food Sci Emer Technol. 2017;42:165–172. doi: 10.1016/j.ifset.2017.06.015. [DOI] [Google Scholar]
- Rostami FM, Mousavi H, Mousavi MRN, Shahsafi M. Efficacy of probiotics in prevention and treatment of infectious diseases. Clin Microbiol Newsl. 2018;40:97–103. doi: 10.1016/j.clinmicnews.2018.06.001. [DOI] [Google Scholar]
- Sabo SS, Vitolo M, González JMD, Oliveira RPS. Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Res Int. 2014;64:527–536. doi: 10.1016/j.foodres.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Salminen S, Von Wright A, editors. Lactic acid bacteria. New York: Marcel Decker; 1993. [Google Scholar]
- Savedboworn W, Niyomrat S, Naknovn J, Phattayakornb K. Impact of inulin on viability and storage stability of probiotic Lactobacillus plantarum TISTR 2075 in fermented rice extract. Agric Nat Resour. 2017;51:1–7. [Google Scholar]
- Sheehan VM, Ross P, Fitzgerald GF. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov Food Sci Emer Technol. 2007;8:279–284. doi: 10.1016/j.ifset.2007.01.007. [DOI] [Google Scholar]
- Shori AB. Influence of food matrix on the viability of probiotic bacteria: a review based on dairy and non-dairy beverages. Food Biosci. 2016;13:1–8. doi: 10.1016/j.fbio.2015.11.001. [DOI] [Google Scholar]
- Stone H, Sidel JL. Sensory evaluation practices. Orlando: Academic Press; 1985. pp. 56–59. [Google Scholar]