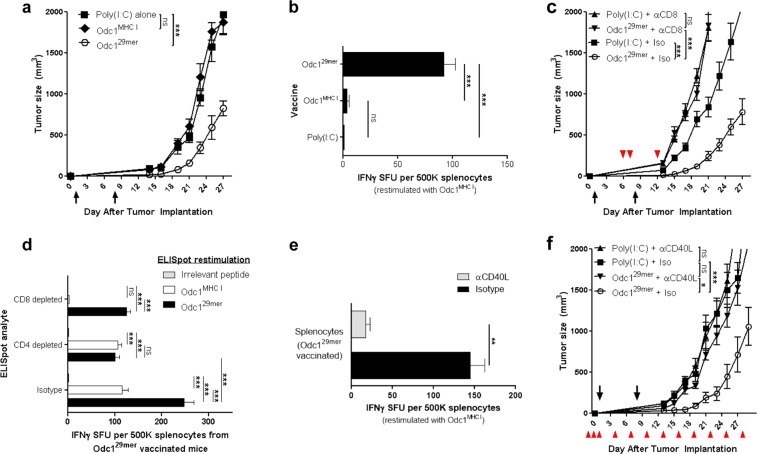
Fig. 1. An endogenous helper epitope facilitates the MHC I neoepitope-mediated therapeutic effects of the Odc1 SLP vaccine.
a Subcutaneous SMA560 tumor growth in mice (n = 7) following therapeutic immunization on days 1 and 8 with poly(I:C) alone, Odc1MHC I, or Odc129mer SLP. b IFNγ ELISpot: splenocyte response to Odc1MHC I 7 days following immunization with poly(I:C) alone, Odc1MHC I, or Odc129mer SLP (n = 3). One-way ANOVA with post hoc Tukey’s test. c Subcutaneous SMA560 tumor growth in mice (n = 7) following therapeutic immunization on days 1 and 8 with poly(I:C) alone or Odc129mer SLP in the context of CD8+ depleting antibody or isotype control (antibody administration denoted by arrowheads). d IFNγ ELISpot: Odc129mer-mediated immune response to the Odc1MHC I-restricted neoepitope or 29mer immunizing peptide in the context of CD8-depletion, CD4-depletion, or isotype-treated control (n = 3). Two-way ANOVA with Bonferroni post hoc test. e IFNγ ELISpot: splenocyte response to Odc1MHC I 7 days following immunization with Odc129mer SLP in the context of CD40L-blocking antibody or isotype control (n = 3). Isotype vs. αCD40L, two-sample t test. f Subcutaneous SMA560 tumor growth in mice (n = 7) following therapeutic immunization on days 1 and 8 with poly(I:C) alone or Odc129mer in the context of CD40L-blocking antibody or isotype control (antibody administration denoted by arrowheads). For tumor growth data, error bars = mean ± s.e.m.; for ELISpot data, errors bars = mean ± s.d.