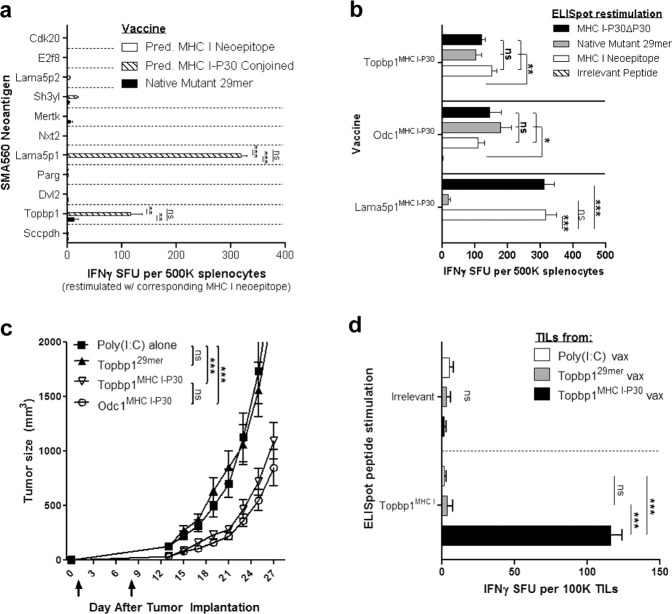
Fig. 3. Conjoined P30 can improve the therapeutic effects of neoantigen vaccines.
a IFNγ ELISpot: splenocyte response to the corresponding putative MHC I-restricted neoepitope 7 days following immunization with the predicted MHC I-restricted neoepitope alone, the predicted MHC I-restricted neoepitope conjoined to P30, or the 29mer SLP spanning the endogenous neoantigen sequence (n = 3). One-way ANOVA with post hoc Tukey’s test. b IFNγ ELISpot: mice (n = 3) were immunized with Topbp1MHC I-P30, Odc1MHC I-P30, or Lama5MHC I-P30 in order to induce a neoepitope-specific CD8+ T-cell response. The ability of the MHC I neoepitope, MHC I-P30, or the native 29mer peptide to restimulate neoepitope-specific CD8+ T cells was assessed 7 days following immunization. The response to P30 alone was subtracted from the MHC I-P30 response (MHC I-P30ΔP30). One-way ANOVA with post hoc Tukey’s test. c Subcutaneous SMA560 tumor growth in mice (n = 7) following therapeutic immunization on days 1 and 8 with poly(I:C) alone, Topbp129mer, Topbp1MHC I-P30, or Odc1MHC I-P30 SLP. d IFNγ ELISpot: evaluation of Topbp1MHC I-reactive TILs within day 27 subcutaneous SMA560 tumors (n = 5-6) from poly(I:C), Topbp129mer, or Topbp1MHC I-P30 vaccinated mice (cells alone-background subtracted). One-way ANOVA with post hoc Tukey’s test. For tumor growth data, error bars = mean ± s.e.m.; for ELISpot data, errors bars = mean ± s.d.