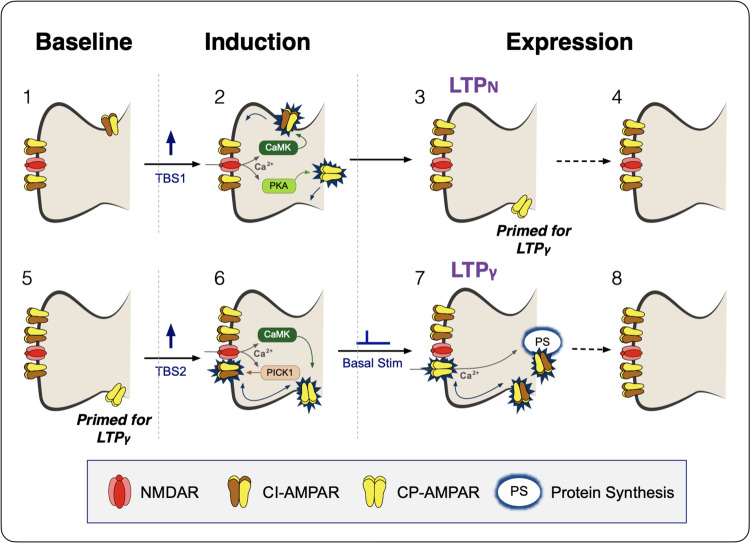
Fig. 8. Schematic outlining the induction of two mechanistically distinct forms of LTP.
1 Under baseline conditions synaptic transmission is mediated by GluA2-containing, calcium-impermeable (CI)-AMPARs, two shown for simplicity. 2 The first theta-burst stimulation (TBS) activates NMDA receptors (NMDARs) and this drives more CI-AMPARs into the synapse by lateral diffusion from a peri-synaptic pool, via a process that involves CaMKII. PKA is also activated (via adenyl cyclase, not shown) and this induces the process of inserting GluA2-lacking calcium-permeable (CP)-AMPARs into peri-synaptic sites on the plasma membrane. 3 LTP is expressed by the increase in number of CI-AMPARs (LTPN) but synapses also become primed for LTPγ by the availability of peri-synaptic CP-AMPARs. 4 Within ~1 h, the peri-synaptic CP-AMPARs are removed and, presumably, degraded. 6 If a second TBS is delivered whilst the synapses are still primed (5) then NMDAR activation drives the peri-synaptically located CP-AMPARs into the synapse, via a CaMKII-dependent process. This might involve an exchange of CP-AMPARs for CI-AMPARs, which are removed from the synapse via a mechanism triggered by PICK1. 7 These CP-AMPARs increase synaptic strength due to their higher single channel conductance (LTPγ). However, their dwell time in the synapses is quite short (~15 min) before they are removed. If synapses remain active, such as by basal stimulation, activation of the transiently available, synaptic CP-AMPARs triggers protein synthesis and the insertion of more CI-AMPARs (8), which can extend the expression of LTP for long periods.