Abstract
Male hypogonadism and major comorbidities such as type 2 diabetes mellitus, obesity, cardiovascular disease, and osteoporosis appear closely connected, forming a vicious cycle that leads to further hypogonadism. This narrative review provides a comprehensive overview of the current literature on the overall burden of male hypogonadism alongside related comorbidities, and how this may be alleviated through testosterone therapy. Observational and clinical data demonstrate that the interaction of male hypogonadism and its related comorbidities is associated with increased mortality, cardiovascular event risk and reduced quality of life. Evidence from epidemiological and registry-based studies shows that this clinical and humanistic burden translates to increased economic burden on health-care systems, through increased physician visits, medical claims, and drug costs. Male hypogonadism can be managed with testosterone therapy, which is intended to normalize testosterone concentrations and thereby reduce both hypogonadism symptoms and risk of comorbidities. Clinical and observational data suggest that in males with hypogonadism, testosterone therapy rapidly and sustainably improves glycemia, reduces risk of progression to diabetes, leads to significantly reduced waist circumference and fat mass, while providing significant positive effects on cardiovascular event risk and bone density. Significant and sustained improvement in patient-reported erectile function, urinary function, and aging male symptoms have also been shown. Economic evaluations have estimated that reduced comorbidity risk following testosterone therapy may lead to cost-savings, with one study estimating yearly inpatient savings of £3732 for treating comorbidities after intervention. A major unmet need exists in the area of male hypogonadism, particularly related to common comorbidities. Options for treatment include testosterone therapy, which has been shown to alleviate the clinical, economic, and humanistic burden associated with these conditions. As the prevalence of male hypogonadism is likely to increase globally, and this condition may be currently underdiagnosed, cost-saving testosterone therapies should be increasingly considered to manage hypogonadism.
Keywords: hypogonadism, testosterone, type 2 diabetes, obesity, cardiovascular disease, burden, cost
Introduction
Hypogonadism in men is characterized by decreases in testicular function and testosterone production, which may lead to lower urinary tract symptoms and erectile dysfunction.1,2 As aging in men is associated with an increased risk of comorbidities that contribute to reduced testosterone,3 hypogonadism becomes increasingly prevalent in men aged over 40 or 50 years;4 however, this condition may be underdiagnosed in practice.5
In addition to its characteristic symptoms, male hypogonadism also appears to be associated with major comorbidities (such as type 2 diabetes mellitus (T2DM), obesity, cardiovascular disease, and osteoporosis), and vice versa.6–8 While hypogonadism may increase deposition of adipose tissue, leading to increased risk of metabolic syndrome, subsequent conditions such as obesity and T2DM may also impair functioning of the hypothalamic-pituitary-gonadal axis, leading to further hypogonadism—a vicious cycle.9,10
Adding to the complexity of disease, underlying etiology of hypogonadism may be closely linked with associated comorbidities.11,12 Secondary hypogonadism, defined as pituitary–hypothalamic failure that is characterized by low testosterone and low or normal gonadotropins, has been strongly associated with obesity and comorbid conditions associated with aging.11,12 In contrast, primary hypogonadism, defined as testicular failure that is characterized by low testosterone and elevated gonadotropins, has been associated with advanced age, which may also be associated with independently occurring comorbidities that further exacerbate hypogonadism.11,12 Although the links between the type of hypogonadism and comorbidities are not fully elucidated, hypogonadism is known to be more common among men with diabetes or obesity, and low testosterone is associated with increased risk of these conditions. Estimated prevalence of hypogonadism varies widely (between 2.1% and 38.7%) in middle-aged and older men,13,14 but appears to increase to approximately 50% among individuals with diabetes or obesity.14 Low total testosterone levels are associated with considerably increased risk of incident T2DM (with odds ratios from 1.6 with <16 nmol/L to 4.5 with <8 nmol/L),15 and total testosterone below 8.7 nmol/L is associated with significantly increased weight circumference and risk of cardiovascular disease-related mortality.16
Hypogonadism and major comorbidities, therefore, appear closely connected. In addition, their interaction appears to lead to a significant burden on individuals and health-care systems. While recent reviews have examined associations between hypogonadism and specific comorbidities,9,17,18 this narrative review provides a comprehensive overview of the topic, and summarizes the current literature on the overall burden of male hypogonadism alongside a broad range of related comorbidities, and how this burden may be alleviated through testosterone therapy.
Burden of Male Hypogonadism and Related Comorbidities
Within the published literature, male hypogonadism and its related comorbidities appear associated with poor clinical outcomes,19–23 increased economic burden on health-care systems,24–26 and burdensome patient-reported symptoms.27–29 Specific to comorbidities, limited data were available on the burden of obesity and T2DM in patients with male hypogonadism, which may be associated with the close link between these diseases.
Increased Mortality and Cardiovascular Event Risk
Observational data demonstrate that the interaction of male hypogonadism and its related comorbidities is associated with poor clinical outcomes.19–23 For example, in a prospective study in the UK (n=857), men with both T2DM and untreated low total or free testosterone (≤12 nmol/L or ≤0.25 nmol/L, respectively) had significantly higher mortality over four years, relative to those with T2DM and normal testosterone (HR: 0.62), or those with T2DM who received treatment for low testosterone (HR: 0.38).19 In another registry study of men with T2DM in the UK (n=581), mortality rate was significantly higher over approximately six years among men with low total testosterone (≤10.4 nmol/L): 17% vs 9% (p=0.003),20 suggesting that the interaction of these two related conditions leads to a substantial clinical burden.
Lowered testosterone also appears to lead to worsened clinical outcomes among men with existing cardiovascular conditions. In a study of consecutive patients with acute ischemic stroke at a single center in Denmark (n=144), post-stroke mortality was significantly associated with low testosterone: at six months, free and total testosterone were significantly higher in surviving patients: 14.7 vs 10.1 nmol/L, and 13.2 vs 10.5 pmol/L respectively (both p=0.004).21 In a Greek study of men with hypertension (n=228), with mean follow-up of 44 months, risk of major adverse cardiovascular events was higher in those with low testosterone levels: in the age-adjusted analysis, this hazard ratio was 2.07 for those with total testosterone 13.9–17.0 nmol/L (not significant), and 3.83 for those with total testosterone below 13.9 nmol/L (p<0.05).22
Poor outcomes are also seen among men with another hypogonadism-related complication (n=1687). In a further Italian cohort study of men with erectile dysfunction, total testosterone below 8 nmol/L was associated with significant increased risk of fatal major adverse cardiovascular events: a hazard ratio of 7.1 (p<0.001).23
Increased Health-care Resource Use
The established clinical burden of male hypogonadism and comorbidities also appears to translate into increased economic burden on health-care systems, through increased physician visits, medical claims, and drug costs.24–26
In a large epidemiological study conducted in Germany (n=2023), men with total testosterone in the lowest decile (7.84 nmol/L on average) had a significantly higher number of outpatient visits per year (p<0.05), and significantly higher yearly outpatient costs (p<0.05), relative to men with total testosterone in the middle eight deciles (16.28 nmol/L on average).24
Comorbidity-related costs appear to be a major contributor to this overall burden. In an analysis of a US insurance database (n=4269), total yearly costs related to cardiovascular comorbidities were almost doubled in men with hypogonadism, relative to those without: $1453 vs $767 (p=0.0001).25 In this analysis, total comorbidity-related costs (comprising medical claim costs and prescription drug costs) were greater than hypogonadism-related costs,25 demonstrating that a considerable financial burden can result both from hypogonadism itself, and from the comorbidities that may be caused by this condition.
Hypogonadism also appears to be associated with increased resource use following surgery. In a large US registry study of men undergoing total knee arthroplasty (n=8393), men with hypogonadism had significantly greater odds of developing medical complications in the 90 days following surgery (OR: 2.12, p<0.0001), significantly greater odds of receiving revision surgery within two years (OR: 1.89, p<0.0001), and significantly longer length of stay (6.11% increase; p=0.02).26 These factors led to significantly increased total cost over the 90 days following surgery, relative to men without hypogonadism ($15,564 vs $14,856; p=0.018).26
Burdensome Patient-reported Symptoms
In addition to its overall clinical and economic burden, male hypogonadism is also associated with negative impacts on individual patients’ experiences, through burdensome patient-reported symptoms,27,28 and reduced quality of life.29
In a prospective study of men in Taiwan (n=680), risk of erectile dysfunction (defined as scoring ≤7 on the International Index of Erectile Function) was significantly increased in men with low testosterone: ×2.4 with total testosterone <11 nmol/L, and ×3.0 with free testosterone <0.23 nmol/L (p<0.05).27 In a further cross-sectional study in South Korea (n=50), lower total testosterone was significantly correlated with worsened urinary symptoms (as measured by the International Prostate Symptom Score; p<0.05).28
Beyond these symptoms, hypogonadism is also known to reduce quality of life: a significant association has been found between low free testosterone and worsened quality of life in the physical, social, and vitality domains of the Short Form (36) Health Survey in a sample of men with T2DM in the UK (p<0.05; n=355),29 thereby demonstrating the overall negative impact of hypogonadism on important aspects of patients’ lives.
Benefits of Testosterone Therapy
Male hypogonadism can be managed with testosterone therapy,30 which is intended to normalize testosterone concentrations to a healthy level and thereby reduce both hypogonadism symptoms and risk of comorbidities.31 This type of therapy is most commonly administered by intramuscular injection in cypionate, enanthate, or undecanoate formulations, or transdermally, with oral, nasal, and buccal preparations also being available.30 It is recommended by the Endocrine Society in hypogonadal men to induce and maintain secondary sex characteristics and correct symptoms of testosterone deficiency.11
Evidence from the published literature suggests that the impacts of male hypogonadism and its related comorbidities can be alleviated through this treatment, which can improve both clinical parameters and patient-reported outcomes,32–39 while reducing overall costs.40
Improved Glycemia, Body Composition, and Cardiovascular Event Risk
Multiple clinical benefits have been seen in clinical and observational studies of testosterone therapy. These include improvements in parameters of T2DM.32–35 In individual clinical trials in hypogonadal men with T2DM (n=199 and n=55), treatment with testosterone undecanoate has been found to reduce HbA1c levels rapidly and sustainably: levels were significantly lowered (vs placebo) at six weeks and at one year (p=0.007 and p<0.001, respectively).33,35 Similarly, in a meta-analysis of 48 placebo-controlled trials examining outcomes of testosterone therapy among varied male populations, parenteral and transdermal testosterone formulations were found to significantly reduce glycemia (p<0.01).34
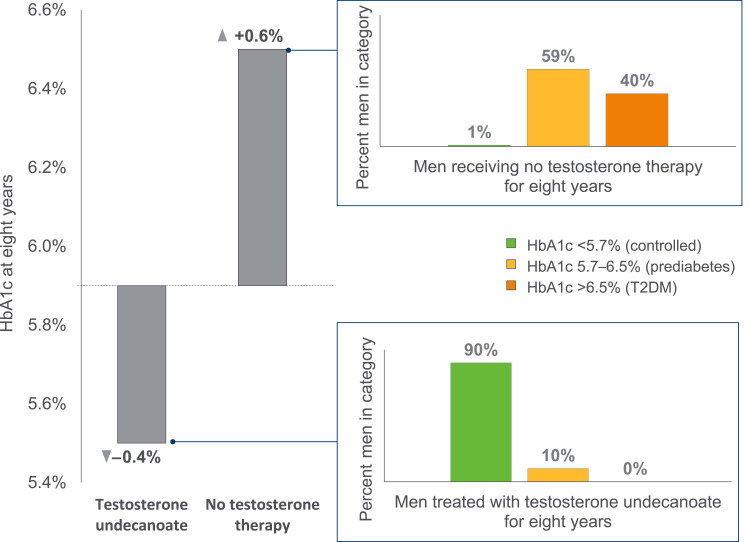
Improvements in glycemic control have also been demonstrated in longer-term observational data from a registry study of hypogonadal men with prediabetes (n=316), where HbA1c was shown to be significantly improved at eight years with testosterone undecanoate treatment (−0.39 ± 0.03%; p<0.0001), and significantly worsened with no treatment (+0.63 ± 0.1%; p<0.0001).32 In addition, hypogonadal men with prediabetes who received testosterone undecanoate treatment were completely prevented from progressing to overt T2DM by the time of last follow-up (approximately six years, on average), while 40.2% of untreated patients had progressed to this state (defined as HbA1c >6.5%);32 with testosterone undecanoate, 89.5% had achieved normal glycemia (HbA1c <5.7%) vs 1.2% with no treatment (Figure 1).32
Figure 1.
Change in HbA1c percentage, and final HbA1c category, of men with hypogonadism within a German registry analysis over eight years, with or without testosterone therapy. Data from Yassin et al.32
Similar improvements are seen in obesity-related parameters following testosterone therapy.33,34,41,42 In clinical trials in men with hypogonadism and in hypogonadal men with T2DM (n=199 and n=60), testosterone undecanoate significantly reduced waist circumference in the short term (30 weeks; p=0.023), and led to further significant reduction in the medium term (36 months; p<0.001).33,41 In meta-analyses of results of clinical trials conducted in varied male populations, treatment with parenteral or transdermal testosterone was also found to lead to significant reduction in fat mass, and increase in fat-free mass (p<0.0001 and p<0.001, respectively).34,42
Further benefits have also been reported in cardiovascular disease, and in parameters related to osteoporosis. Within a German observational study (n=805), data collected over 12 years showed significantly lower risks of myocardial infarction and stroke in men with hypogonadism treated with testosterone undecanoate, relative to those who were untreated: 0% in men who were treated, vs 15.0% and 17.8% in men who were untreated (p<0.0001).38 In a clinical trial in hypogonadal men with metabolic syndrome (n=60), testosterone undecanoate treatment was shown to increase femoral and lumbar bone density by ~5% each year, and to lead to significantly superior density at two and three years relative to no treatment (p<0.005).41
Cost-effective Improvement, and Cost Savings
Through its beneficial impact on clinical parameters and risk of comorbidity, testosterone therapy also appears to be a cost-effective and cost-saving intervention. Economic models in Sweden and the UK have demonstrated the benefits of testosterone therapy on health-care systems as a whole, through its effect of reducing comorbidity risk.40,43
In Sweden, lifetime treatment of male hypogonadism with testosterone undecanoate was shown to be cost-effective, with an incremental cost-effectiveness ratio (ICER) of €19,720 (in 2009 euros) per quality-adjusted life year, vs no treatment.43 This model incorporated the effects of testosterone undecanoate treatment on reducing risks of cardiovascular events, T2DM, fractures, depression, and death in hypothetical sample of 100,000 men with late-onset hypogonadism.43 A one-way sensitivity analysis that altered the risk of comorbidities, drug cost and age, confirmed the ICER results were robust, with changes to the risk of T2DM causing the most variation. The primary drivers of increased cost were the cost of testosterone undecanoate treatment itself, and the greater indirect cost associated with reduced mortality risk (and therefore longer survival) within the treated population; however, these increased costs were partially compensated by the reduced cost of treating comorbidities and complications.43
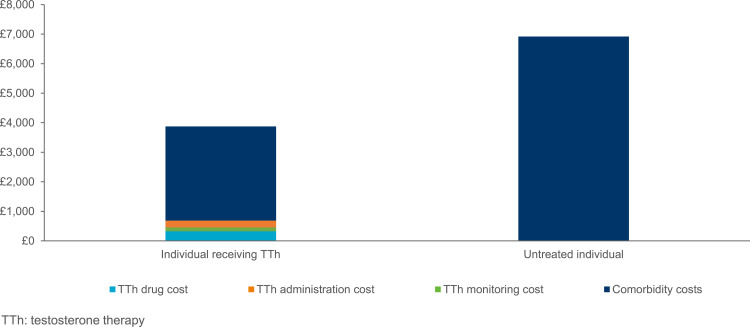
In the UK, treatment of male hypogonadism in males aged ≥40 with intramuscular testosterone undecanoate was also shown to have a positive cost impact, vs no treatment over a 10-year time horizon.40 This analysis included resource use and unit costs only for patients treated with IM testosterone undecanoate and calculated average direct annual costs of managing each comorbidity from the NHS national tariff in both scenarios. Across all comorbidities, a total of 408,481 patients were estimated to receive treatment with testosterone undecanoate and 913,676 patients did not receive testosterone therapy over 10 years. Overall, additional costs of drug, administration, and monitoring were outweighed by reductions in the inpatient cost of managing major comorbidities (T2DM, obesity, CVD, and osteoporosis).40 Specifically, individual yearly testosterone therapy costs of £687 were outweighed by a £3732 reduction in the yearly inpatient cost of treating comorbidities, of which approximately half was accounted for by cardiovascular disease-related cost savings (£1727) [Figure 2].40 However, this was considered to be an underestimation given possible interaction between comorbidities were not examined and the costs of drugs used to manage comorbidities were not included in this analysis.
Figure 2.
Yearly cost of inpatient management of hypogonadism-related comorbidities, in an individual treated or untreated with testosterone therapy (TTh).
Improvements in Patient-reported Symptoms and Quality of Life
In addition to its clinical and economic benefits, testosterone therapy has also been shown to improve individual patients’ experience, through reduction in patient-reported symptom burden, and improvement in quality of life.36–39
In a clinical trial (n=261), treatment of male hypogonadism with testosterone undecanoate was shown to lead to improvements in erectile dysfunction (by IIEF-5), and significant improvements in urinary function (by IPSS) and aging male symptoms (by AMS) at three months (p<0.05).36 These effects are confirmed by German registry data from men with hypogonadism (n=656) showing year-on-year improvements in these three parameters over eight years of treatment, and significant superiority (vs no treatment) at each timepoint from two to eight years (p<0.0001).39 The patient-reported effect on urinary function was also confirmed by significantly superior post-void bladder volume with testosterone undecanoate treatment, vs no treatment, at the same timepoints.39 In another analysis of registry data from men with hypogonadism (n=805), erectile function, urinary function, and aging male symptoms all remained significantly superior with testosterone undecanoate treatment at 10 years (p<0.0001),38 thereby demonstrating the persistence of these benefits into the long-term.
In data from a further clinical trial in men with hypogonadism in Malaysia (n=114), treatment with testosterone undecanoate was also shown to lead to significant improvements in mental health-related quality of life, at 48 weeks of treatment (as measured by the mental health composite score of the Short Form (36) Health Survey; p<0.013),37 demonstrating an improvement in more general quality of life with testosterone therapy.
Limitations of Testosterone Therapy
Despite the clinical and economic evidence supporting the use of testosterone therapy for male hypogonadism, there remain concerns related to the potential risk of prostate cancer that has been reported in certain patient groups.11,44 As a result, guidelines published by the Endocrine Society do not recommend testosterone therapy in men planning fertility, in men with prostate cancer or prostate-specific antigen level >4 ng/mL.11 Acknowledging the benefits of testosterone therapy, shared decision making is advised to ensure patients are aware of the potential prostate cancer risks and monitoring options are offered, particularly in those who are 40 to 69 years old and at increased risk of prostate cancer, such as African Americans and men with a first-degree relative with diagnosed prostate cancer.11
However, observational studies have investigated safety concerns and provided evidence that testosterone therapy does not lead to any increased risk of prostate cancer. In a large multinational registry study of men with hypogonadism (n=999), rates of confirmed prostate cancer per biopsy were comparable with or without testosterone therapy: 37.5% vs 37.0% respectively.45 This finding may address previous concerns relating to prostate cancer risk with testosterone therapy, although further studies are required to support the guideline recommendations.44
Conclusion
A major unmet need exists in the area of male hypogonadism, particularly in relation to the major comorbidities that commonly occur. Symptoms of hypogonadism appear highly prevalent in middle-aged or older men with diabetes or obesity, and low testosterone is associated with increased risk of T2DM, worsened body composition, and increased risk of cardiovascular disease-related mortality. Together, these conditions lead to increased patient-reported symptom burden, increased mortality risk, and increased health care resource use.
As population aging leads to an increase in the proportion of older individuals globally,46 the overall prevalence of comorbidities that increase male hypogonadism risk is likely to increase, and the burden of this condition and its further downstream comorbidities will become greater. There is, therefore, a need to consider the available treatment options for male hypogonadism— especially those that may also provide additional benefits in terms of reducing the burden of resulting comorbidities.
Options for treatment include testosterone therapy, which has been shown to alleviate the clinical, economic, and humanistic burden associated with these conditions. Its benefits include significantly improving glycemia and preventing progression from prediabetes to T2DM, significantly improving body composition and waist circumference, and conferring improvements in patient-reported symptoms that persist into the long-term. Limitations include the monitoring of prostate parameters that may be necessary during treatment,47 and the potential negative effect on male fertility.48
As the prevalence of male hypogonadism is likely to increase globally, and this condition may be currently underdiagnosed, cost-effective and cost-saving testosterone therapies should be increasingly considered for use in order to manage this condition.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgment
Bayer markets a commonly used testosterone therapy.
Funding Statement
Adelphi Values PROVE were commissioned by Bayer to conduct and report this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Sandy Yeo is an employee of Bayer, Singapore. Katsiaryna Holl, Nicolás Peñaherrera and Ulrike Wissinger are employees of Bayer, Germany. Kate Anstee and Robin Wyn are employees of Adelphi Values PROVE, who were commissioned by Bayer to conduct and report this research. The authors report no other conflicts of interest in this work.
References
- 1.Rastrelli G, Vignozzi L, Corona G, et al. Testosterone and Benign Prostatic Hyperplasia. Sex Med Rev. 2019;7(2):259–271. doi: 10.1016/j.sxmr.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 2.Traish AM. Negative Impact of Testosterone Deficiency and 5alpha-Reductase Inhibitors Therapy on Metabolic and Sexual Function in Men. Adv Exp Med Biol. 2017;1043:473–526. [DOI] [PubMed] [Google Scholar]
- 3.Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737–2745. doi: 10.1210/jc.2007-1972 [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Gooren LJ. Late onset hypogonadism of men is not equivalent to the menopause. Maturitas. 2014;79(1):52–57. doi: 10.1016/j.maturitas.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 5.Defeudis G, Mazzilli R, Gianfrilli D, et al. The CATCH checklist to investigate adult-onset hypogonadism. Andrology. 2018;6(5):665–679. doi: 10.1111/andr.12506 [DOI] [PubMed] [Google Scholar]
- 6.Traish AM, Guay A, Feeley R, et al. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30(1):10–22. doi: 10.2164/jandrol.108.005215 [DOI] [PubMed] [Google Scholar]
- 7.Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl. 2009;30(1):23–32. doi: 10.2164/jandrol.108.005751 [DOI] [PubMed] [Google Scholar]
- 8.Traish AM, Saad F, Feeley RJ, et al. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl. 2009;30(5):477–494. doi: 10.2164/jandrol.108.007245 [DOI] [PubMed] [Google Scholar]
- 9.Grossmann M, Ng Tang M. Late‐onset hypogonadism: metabolic impact. J Androl. 2019;1:25. [DOI] [PubMed] [Google Scholar]
- 10.De Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients. 2018;10(4):474. doi: 10.3390/nu10040474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715–1744. doi: 10.1210/jc.2018-00229 [DOI] [PubMed] [Google Scholar]
- 12.Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95(4):1810–1818. doi: 10.1210/jc.2009-1796 [DOI] [PubMed] [Google Scholar]
- 13.Zarotsky V. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J Hormones. 2014;1:2014. [Google Scholar]
- 14.Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762–769. doi: 10.1111/j.1742-1241.2006.00992.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schipf S, Haring R, Friedrich N, et al. Low total testosterone is associated with increased risk of incident type 2 diabetes mellitus in men: results from the Study of Health in Pomerania (SHIP). Aging Male. 2011;14(3):168–175. doi: 10.3109/13685538.2010.524955 [DOI] [PubMed] [Google Scholar]
- 16.Haring R, Volzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J. 2010;31(12):1494–1501. doi: 10.1093/eurheartj/ehq009 [DOI] [PubMed] [Google Scholar]
- 17.Gambineri A, Pelusi C. Sex hormones, obesity and type 2 diabetes: is there a link? Endocrine Connections. 2019;8(1):R1–R9. doi: 10.1530/EC-18-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrageta DF, et al. Obesity and male hypogonadism: tales of a vicious cycle. Obesity Reviews. 2019;20(8):1148–1158. [DOI] [PubMed] [Google Scholar]
- 19.Hackett G, Heald AH, Sinclair A, et al. Serum testosterone, testosterone replacement therapy and all‐cause mortality in men with type 2 diabetes: retrospective consideration of the impact of PDE5 inhibitors and statins. Int J Clin Pract. 2016;70(3):244–253. doi: 10.1111/ijcp.12779 [DOI] [PubMed] [Google Scholar]
- 20.Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinology. 2013;169(6):725–733. doi: 10.1530/EJE-13-0321 [DOI] [PubMed] [Google Scholar]
- 21.Jeppesen LL, Jørgensen HS, Nakayama H, et al. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol. 1996;16(6):749–754. doi: 10.1161/01.ATV.16.6.749 [DOI] [PubMed] [Google Scholar]
- 22.Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, et al. Plasma total testosterone and incident cardiovascular events in hypertensive patients. Am J Hypertens. 2013;26(3):373–381. doi: 10.1093/ajh/hps056 [DOI] [PubMed] [Google Scholar]
- 23.Corona G, Monami M, Boddi V, et al. Low testosterone is associated with an increased risk of MACE lethality in subjects with erectile dysfunction. J Sex Med. 2010;7(4):1557–1564. doi: 10.1111/j.1743-6109.2009.01690.x [DOI] [PubMed] [Google Scholar]
- 24.Haring R, Baumeister SE, Völzke H, et al. Prospective association of low serum total testosterone levels with health care utilization and costs in a population‐based cohort of men. Int J Androl. 2010;33(6):800–809. doi: 10.1111/j.1365-2605.2009.01029.x [DOI] [PubMed] [Google Scholar]
- 25.Kaltenboeck A, Foster S, Ivanova J, et al. The direct and indirect costs among US privately insured employees with hypogonadism. J Sex Med. 2012;9(9):2438–2447. doi: 10.1111/j.1743-6109.2012.02810.x [DOI] [PubMed] [Google Scholar]
- 26.Ardeljan AD, Meneses ZA, Neal BV, et al. Increased medical complications, revisions, in-hospital lengths of stay, and cost in patients with hypogonadism undergoing primary total knee arthroplasty. J Arthroplasty. 2020;35(1):95–99. doi: 10.1016/j.arth.2019.08.025 [DOI] [PubMed] [Google Scholar]
- 27.Hwang T, Lo H-C, Tsai T-F, et al. Association among hypogonadism, quality of life and erectile dysfunction in middle-aged and aged male in Taiwan. Int J Impot Res. 2007;19(1):69–75. doi: 10.1038/sj.ijir.3901480 [DOI] [PubMed] [Google Scholar]
- 28.Shim JS, Kim JH, Yoon YS, et al. Serum testosterone levels are negatively correlated with international prostate symptom score and transitional prostate volume. Low Urin Tract Symptoms. 2018;10(2):143–147. doi: 10.1111/luts.12150 [DOI] [PubMed] [Google Scholar]
- 29.Walter D, et al. Low testosterone is associated with poor health-related quality of life in men with Type 2 diabetes: P162. Diabetic Medicine. 2011;28. [Google Scholar]
- 30.Thirumalai A, Berkseth KE, Amory JK. Treatment of Hypogonadism: current and Future Therapies. F1000Research. 2017;6:68. doi: 10.12688/f1000research.10102.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surampudi P, Swerdloff RS, Wang C. An update on male hypogonadism therapy. Expert Opin Pharmacother. 2014;15(9):1247–1264. doi: 10.1517/14656566.2014.913022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yassin A, Haider A, Haider KS, et al. Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: eight-year data from a registry study. Diabetes Care. 2019;42(6):1104–1111. doi: 10.2337/dc18-2388 [DOI] [PubMed] [Google Scholar]
- 33.Hackett G, Cole N, Bhartia M, et al. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11(3):840–856. doi: 10.1111/jsm.12404 [DOI] [PubMed] [Google Scholar]
- 34.Corona G, Giagulli VA, Maseroli E, et al. Testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinology. 2016;174(3):R99–R116. doi: 10.1530/EJE-15-0262 [DOI] [PubMed] [Google Scholar]
- 35.Groti K, Žuran I, Antonič B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21(3):158–169. doi: 10.1080/13685538.2018.1468429 [DOI] [PubMed] [Google Scholar]
- 36.Almehmadi Y, Yassin AA, Nettleship JE, et al. Testosterone replacement therapy improves the health-related quality of life of men diagnosed with late-onset hypogonadism. Arab J Urology. 2016;14(1):31–36. doi: 10.1016/j.aju.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong S-F, Ng C-J, Lee B-C, et al. Effect of long-acting testosterone undecanoate treatment on quality of life in men with testosterone deficiency syndrome: a double blind randomized controlled trial. Asian J Androl. 2012;14(4):604. doi: 10.1038/aja.2011.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saad F, Caliber M, Doros G, et al. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male. 2020;23(1):81–92. doi: 10.1080/13685538.2019.1575354 [DOI] [PubMed] [Google Scholar]
- 39.Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199(1):257–265. doi: 10.1016/j.juro.2017.07.039 [DOI] [PubMed] [Google Scholar]
- 40.Spencer C, Anstee K, Yeo S, et al. PIH8: understanding the economic burden of comorbidities associated with male hypogonadism: a cost model in England. Value in Health. 2020;23:S152. doi: 10.1016/j.jval.2020.04.404 [DOI] [Google Scholar]
- 41.Aversa A, Bruzziches R, Francomano D, et al. Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male. 2012;15(2):96–102. doi: 10.3109/13685538.2011.631230 [DOI] [PubMed] [Google Scholar]
- 42.Skinner JW, et al. Muscular responses to testosterone replacement vary by administration route: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2018;9(3):465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arver S, Luong B, Fraschke A, et al. Is testosterone replacement therapy in males with hypogonadism cost‐effective? An analysis in Sweden. J Sex Med. 2014;11(1):262–272. doi: 10.1111/jsm.12277 [DOI] [PubMed] [Google Scholar]
- 44.Eisenberg ML. Testosterone replacement therapy and prostate cancer incidence. World J Men’s Health. 2015;33(3):125–129. doi: 10.5534/wjmh.2015.33.3.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debruyne FM, Behre HM, Roehrborn CG, et al. Testosterone treatment is not associated with increased risk of prostate cancer or worsening of lower urinary tract symptoms: prostate health outcomes in the Registry of Hypogonadism in Men. BJU Int. 2017;119(2):216–224. doi: 10.1111/bju.13578 [DOI] [PubMed] [Google Scholar]
- 46.Seftel A. Male hypogonadism. Part I: epidemiology of hypogonadism. Int J Impot Res. 2006;18(2):115–120. doi: 10.1038/sj.ijir.3901397 [DOI] [PubMed] [Google Scholar]
- 47.Miranda EP, Torres LO. Late-onset hypogonadism: prostate safety. Andrology. 2020;8(6):1606–1613. doi: 10.1111/andr.12772 [DOI] [PubMed] [Google Scholar]
- 48.Patel AS, Leong JY, Ramos L, et al. Testosterone Is a Contraceptive and Should Not Be Used in Men Who Desire Fertility. World J Men’s Health. 2019;37(1):45–54. doi: 10.5534/wjmh.180036 [DOI] [PMC free article] [PubMed] [Google Scholar]