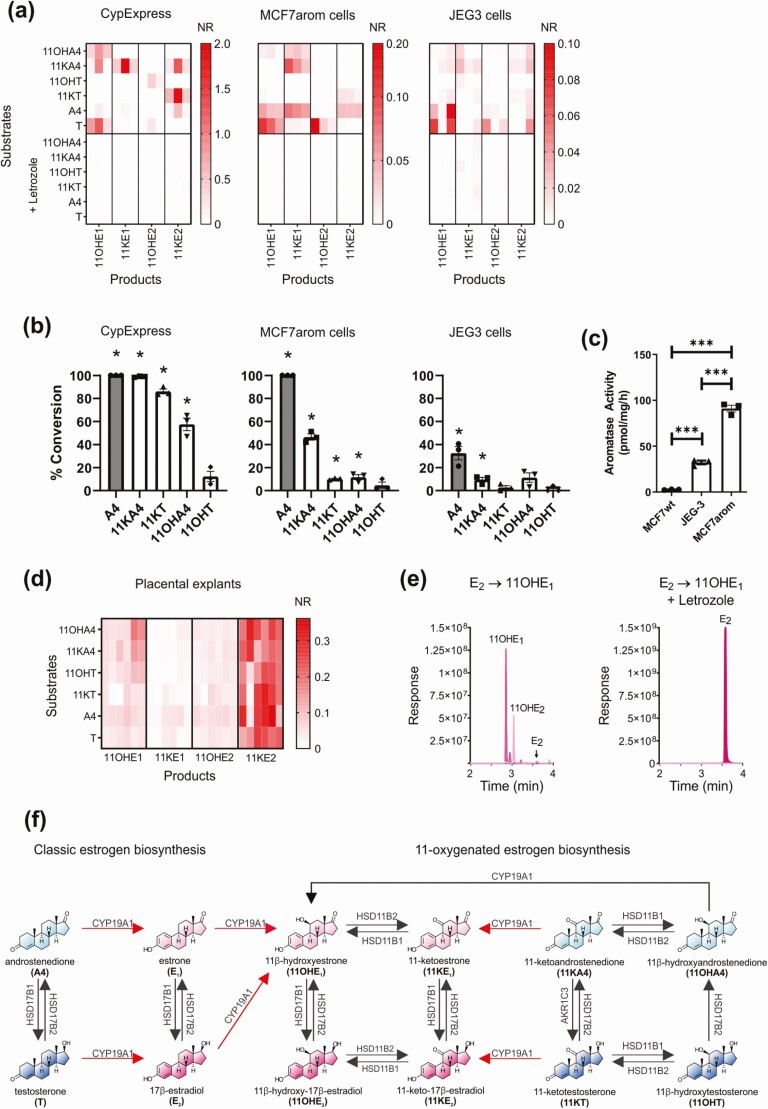
Figure 1.
Biosynthesis of 11-oxygenated estrogens from 11-oxygenated and classic androgens. A, Biosynthesis of 11-oxygenated estrogens by 3 in vitro aromatase expression systems. Assays (n = 3) were performed using 1-µM androgen substrate with and without 10-µM letrozole. Owing to the lack of commercially available 11-oxygenated estrogen standards, conversion data are expressed as the mean normalized responses (NR) from independent experiments each performed in triplicate (NR: [peak area/peak area of the internal standard]/protein). B, Percentage conversion of aromatase substrates as calculated substrate utilization. Data points are shown as mean ± SEM from 3 independent experiments each performed in triplicate. Statistical analysis was performed by comparing each steroid to its substrate specific control. *P less than .05; unpaired t test. C, Aromatase activity in MCF7 (wild type), JEG3, and MCF7arom cells was determined using a tritiated water-release assay using A4 (1β-3H[N]) as substrate. Data represent 3 independent experiments shown as mean ± SEM. ***P less than .001; one-way analysis of variance and Dunnett’s multiple comparisons test. D, Biosynthesis of 11-oxygenated estrogens by human placenta explants (n = 6). E, Representative (multiple reaction monitoring [MRM]) chromatograms demonstrating the aromatase-catalyzed conversion of 1-µM E2 directly to 11OHE1 in the CypExpress system (n = 3). Conversion was inhibited by the addition of 10-µM letrozole. F, Schematic overview of 11-oxygenated estrogen biosynthesis. Androgens and estrogens are shown in blue and pink, respectively. All observed aromatase catalyzed reactions are shown in red. Steroids: 11OHA4, 11β-hydroxyandrostenedione; 11KA4, 11-ketoandrostenedione; 11OHT, 11β-hydroxytestosterone; 11KT, 11-ketotestosterone; 11OHE1, 11β-hydroxyestrone; 11OHE2, 11β-hydroxy-17β-estradiol; 11KE1, 11-ketoestrone; 11KE2, 11-keto-17β-estradiol; A4, androstenedione; E1, estrone; E2, 17β-estradiol; T, testosterone.