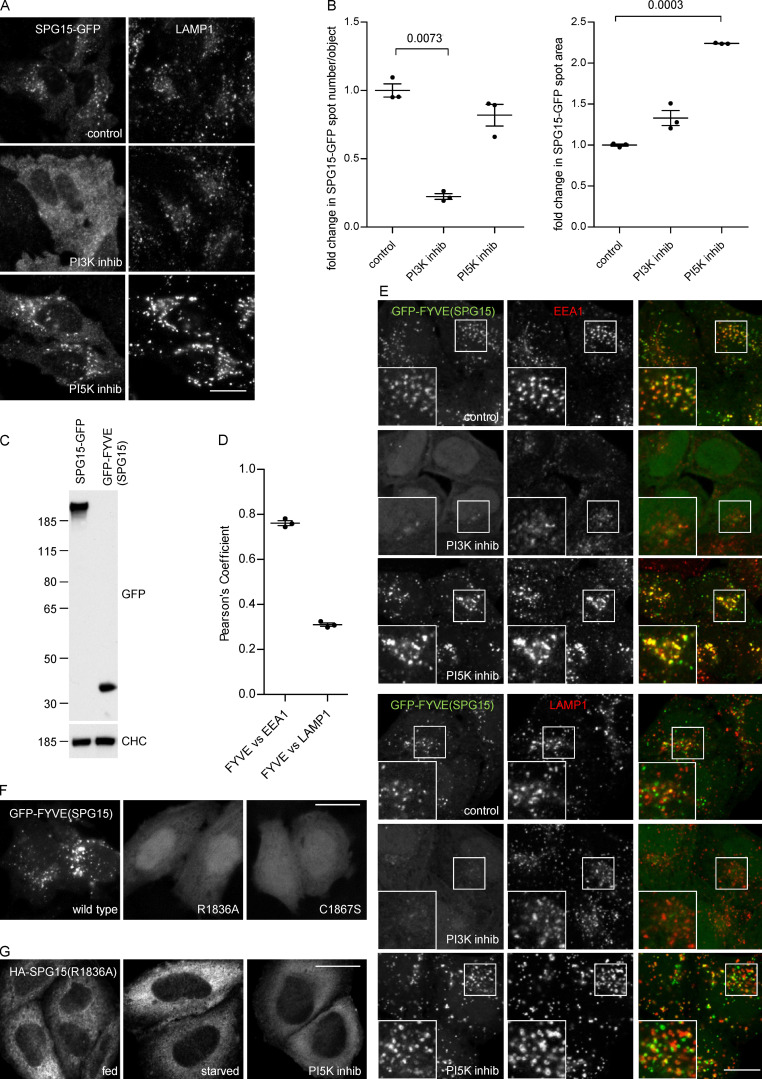
Figure 2.
PI3P binding is necessary for complex recruitment but not sufficient for its localization. (A) IF double labeling of SPG15-GFP (amplified with anti-GFP) and LAMP1 following treatment with either a PI3K inhibitor (100 nM wortmannin; 1 h) or a PI5K inhibitor (1 µM YM201636; 1 h). Treatment with the PI3K inhibitor results in a loss of membrane-associated SPG15-GFP, causing the cells to appear brighter because of increased cytosolic labeling, while treatment with the PI5K inhibitor results in enhanced membrane labeling. Scale bar: 20 µm. (B) Quantification of the effect of PI3K or PI5K inhibitors on membrane recruitment of SPG15-GFP, showing the fold change of either spot number per object or average spot size relative to control. The slight reduction in the number of spots in the cells treated with the PI5K inhibitor may be due to an indirect effect, such as increased clustering and fusion of endosomes and lysosomes (Bissig et al., 2017). The experiment was performed in biological triplicate (mean indicated; n = 3), and 20 cells were quantified per condition. Error bars represent SEM. (C) Western blots of whole-cell lysates from HeLa stable cell lines expressing SPG15-GFP or GFP-FYVE(SPG15), showing similar levels of expression. Clathrin heavy chain (CHC) was used as a loading control. Image is representative of two independent experiments. (D and E) IF double labeling and quantification of GFP-tagged SPG15 FYVE domain [GFP-FYVE(SPG15)], stably expressed in HeLa cells, and either EEA1 or LAMP1. Where indicated, cells were treated with either a PI3K inhibitor (100 nM wortmannin; 1 h) or a PI5K inhibitor (1 µM YM201636; 1 h). Scale bar: 20 µm. The quantification of the colocalization between GFP-FYVE(SPG15) and either EEA1 or LAMP1 was performed using Pearson’s correlation coefficient. The experiment was performed in biological triplicate (mean indicated; n = 3), and 20 cells were quantified per condition. Note that the isolated FYVE domain of SPG15 predominantly colocalizes with the early endosomal marker EEA1. Error bars represent SEM. (F) Localization of GFP-FYVE(SPG15), either wild type, PI3P-binding mutant (R1836A), or zinc-binding mutant (C1867S), transiently expressed in HeLa cells. The inability to bind PI3P or zinc renders the FYVE domain of SPG15 cytosolic. Scale bar: 20 µm. (G) Localization of HA-SPG15 with a PI3P-binding mutation (R1836A) transiently expressed in HeLa cells. Where indicated, cells were treated with either a PI3K inhibitor (100 nM wortmannin; 1 h) or a PI5K inhibitor (1 µM YM201636; 1 h). The inability to bind PI3P renders the full-length SPG15 cytosolic. Scale bar: 20 µm.