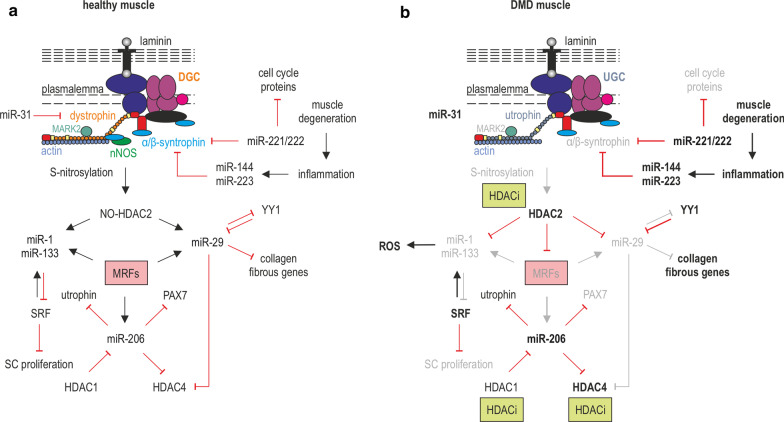
Fig. 7.
Dystrophin-nNOS signaling in epigenetic control of muscle differentiation. a The dystrophin-nNOS signaling regulates the epigenetic profile of myogenesis via S-nitrosylation of HDAC2 (class I HDAC), which affects gene expression through changes in histone acetylation. miR-221/222 are involved in the inhibition of cell cycle proteins and miR-222 targets β-syntrophin, while miR-31 temporarily targets dystrophin. Transcription of miR-1 and miR-133 is controlled by the HDAC2 S-nitrosylation state, regulated by nNOS activity. Also, miR-133 targets SRF during proliferation, which in a self-regulating manner promotes miR-133 expression, and miR-29 is coregulated by the HDAC2 S-nitrosylation state and YY1, while miR-206 is regulated by MRFs and the HDAC1 activity, and supports cell cycle inhibition by targeting PAX7. b In DMD, the epigenetic differentiation network is disturbed due to the absence of dystrophin-α/β-syntrophin-nNOS signaling and interrupted HDAC2 S-nitrosylation. As a consequence, a decrease in the levels of miR-133/1 and miR-29, followed by inhibition of muscle differentiating genes and ROS generation is observed. Also, diminished amounts of miR-29 levels correlate with an increase in collagen and fibrotic tissue. The miR-206 level is higher, resulting in an imbalance between proliferative and differentiated states. Additionally, miR-144 and miR-223 are also observed following an increase in inflammation and muscle degeneration. miRNA and protein names marked in bold and grey indicate their upregulation and downregulation, respectively; black arrows mark activation while red and grey blunt lines inhibition and reduction in inhibition, respectively. HDACi targeting HDACs and the corresponding processes are marked in yellow boxes