Abstract
Background
Antimicrobial resistance (AMR) is an increasing global health concern reducing options for therapy of infections and also for perioperative prophylaxis. Many Enterobacteriaceae cannot be treated anymore with third generation cephalosporins (3GC) due to the production of certain 3GC hydrolysing enzymes (extended spectrum beta-lactamases, ESBLs). The role of animals as carriers and vectors of multi-resistant bacteria in different geographical regions is poorly understood. Therefore, we investigated the occurrence and molecular characteristics of ESBL-producing Escherichia coli (E. coli) in wild birds and slaughtered cattle in Ibadan, Nigeria.
Cattle faecal samples (n = 250) and wild bird pooled faecal samples (cattle egrets, Bubulcus ibis, n = 28; white-faced whistling duck, Dendrocygna viduata, n = 24) were collected and cultured on cefotaxime-eosin methylene blue agar. Antimicrobial susceptibility was determined by agar diffusion assays and all 3GC resistant isolates were genotypically characterised for AMR genes, virulence associated genes (VAGs) and serotypes using DNA microarray-based assays.
Results
All 3GC resistant isolates were E. coli: cattle (n = 53), egrets (n = 87) and whistling duck (n = 4); cultured from 32/250 (12.8%), 26/28 (92.9%), 2/24(8.3%), cattle, egrets and whistling duck faecal samples, respectively. blaCTX-M gene family was prevalent; blaCTX-M15 (83.3%) predominated over blaCTX-M9 (11.8%). All were susceptible to carbapenems. The majority of isolates were resistant to at least one of the other tested antimicrobials; multidrug resistance was highest in the isolates recovered from egrets.
The isolates harboured diverse repositories of other AMR genes (including strB and sul2), integrons (predominantly class 1) and VAGs. The isolates recovered from egrets harboured more AMR genes; eight were unique to these isolates including tetG, gepA, and floR. The prevalent VAGs included hemL and iss; while 14 (including sepA) were unique to certain animal isolates. E. coli serotypes O9:H9, O9:H30 and O9:H4 predominated. An identical phenotypic microarray profile was detected in three isolates from egrets and cattle, indicative of a clonal relationship amongst these isolates.
Conclusion
Wild birds and cattle harbour diverse ESBL-producing E. coli populations with potential of inter-species dissemination and virulence. Recommended guidelines to balance public health and habitat conservation should be implemented with continuous surveillance.
Keywords: ESBL, Microarray, Africa, CTX-M, Wild birds, Cattle
Background
Antimicrobial resistance (AMR) in bacteria is an increasing and widespread public health concern which has grossly eroded the efficacy of many antimicrobial substances. Multidrug resistant – and especially beta-lactam-resistant – bacteria have further limited therapeutic options, leading to treatment failure [1], prolonged stay of patients in hospitals and the need for other and usually more expensive and/or more toxic drugs [2, 3]. Of particular concern are extended-spectrum β-lactamase (ESBL)-producing bacteria which are resistant to third generation cephalosporins (3GC) through hydrolysis of these antimicrobials [4, 5] and which pose especially serious treatment challenges [6]. ESBL genes could spread through clonal dissemination of host bacteria or via mobile genetic elements [7, 8]. ESBL genes of the CTX-M family are the most common with blaCTX-M-1/15 being most prevalent [9]. The prevalence of different types of CTX-M enzymes also varies with geographical areas [10]. The family Enterobacteriaceae, particularly E. coli, are common ESBL-producers. They inhabit animal and human guts and could cause diverse infections [8, 11].
While AMR is a global phenomenon [12], the situation is worsened in the developing countries including Nigeria by lack of antimicrobial regulation policies, unrestricted access and indiscriminate use of antimicrobials, and the lack of national antimicrobial resistance surveillance programmes.
AMR is a complex public health challenge involving humans, animals (wildlife, livestock and companion animals) and the environment [13–18]. Exchange of antimicrobial resistance genes and/or host bacteria can occur between animals and humans; thus, warranting a unified-health approach to its control. Food animals including cattle are reservoirs of ESBL-producing Enterobacteriaceae that could disseminate through the food chain and/or animal contact [8, 19, 20]. Reports on ESBL-producing Enterobacteriaceae in food animals in Africa including Nigeria are scarce [13, 15, 21]. E. coli can be grouped into commensals and pathogenic strains based on the presence of some virulence-associated genes (VAGs); the latter are further divided into pathotypes, harbouring different sets of VAGs and are associated with different infections [22, 23].
The role of wild animals, particular wild birds, as reservoirs and vectors of antimicrobial resistant bacteria is increasingly appreciated. ESBL-producing Enterobacteriaceae have been reported in at least 80 species of wildlife dominated by wild birds, with the most prevalent species being E. coli [18]. However, there are only few reports from Africa [24]. Wild animals are not directly exposed to antibiotics but rather indirectly, through environmental contamination. They could be infected or colonised by resistant bacteria through feeding on contaminated animal wastes or carcasses and drinking of contaminated water. In Nigeria, egrets are common in the South-Western regions (especially during the dry season), and they are often associated with cattle herds, feeding on the cattle ticks and scavenging the faeces. They are also scavengers in urban areas, particularly in abattoirs.
This study determined the occurrence of ESBL-producing Enterobacteriaceae in two populations of wild birds (egrets and whistling ducks) and cattle at slaughter. The genes encoding ESBLs and other antimicrobial resistance genes as well as virulence-associated genes were determined. The serotypes of all E. coli isolates were determined by serogenotyping (https://onlinelibrary.wiley.com/doi/full/10.1111/1348-0421.12120).
Results
A majority (26/28, 92.9%) of the collected egret faecal samples yielded bacterial growth (bacterial colonies) on cefotaxime-eosin methylene blue agar (CEMB) plates; while 32/250 (12.8%) and 2/24 (8.3%) of cattle and whistling duck faecal samples yielded growth, respectively.
From the CEMB plates, 88 (egret), 4 (duck) and 55 (cattle) putative E. coli isolates were picked for further analysis; in addition to one unassigned bacterial isolate each from egret and cattle faecal samples. All the putative E. coli isolates were identified by microarray using the E. coli PanType Kit. The unassigned bacterial isolate from the egrets was identified as Citrobacter freundii and the one from cattle as Salmonella enterica spp. enterica serovar Tees (Table 1).
Table 1.
Overview of all collected animal faecal samples and number of yielded bacterial isolates
| Animals | Collected faecal samples | No. samples yielding bacterial growth on CEMBd |
Total bacterial isolates | E. coli isolates | Non-E. coli isolates | Cefotaxime resistant E. coli isolatesa | Cefotaxime susceptible E. coli isolates | Non-E. coli isolates resistant to cefotaxime |
|---|---|---|---|---|---|---|---|---|
| Cattle | 250 | 32 | 56 | 55 | 1b | 53 | 2 | 0 |
| Egret | 28 | 26 | 89 | 88 | 1c | 87 | 1 | 0 |
| Whistling duck | 24 | 2 | 4 | 4 | 0 | 4 | 0 | 0 |
| Total | 302 | 60 | 149 | 147 | 2 | 144 | 3 | 0 |
a Subjected to further analyses (antimicrobial susceptibility testing to other antimicrobials; microarray-based analysis of antimicrobial and virulence genes and serotypes)
b Salmonella enterica spp. Enterica serovar Tees
c Citrobacter freundii; dCEMB (cefotaxime-eosine methylene blue agar plates)
A total of 87/88 (98.9%) (egret), 4/4 (100%) (whistling duck) and 53/55 (96.4%) (cattle) E. coli isolates exhibited cefotaxime resistance (Tables 1 and 2). The Salmonella and Citrobacter isolates were susceptible to cefotaxime (Table 1).
Table 2.
Distribution of cefotaxime resistant E. coli (CREC) isolates cultured from faecal samples of cattle and wild birdsa, b, c
| Sample codes | No. CRECd | Sample codes | No. CREC | Sample codes | No. CREC | Sample codes | No. CREC |
|---|---|---|---|---|---|---|---|
| cattle 10 | 1 | cattle 136 | 1 | cattle 238 | 2 | egrets 4.6 | 4 |
| cattle 15 | 1 | cattle 138 | 2 | cattle 240 | 1 | egrets 4.7 | 2 |
| cattle 29 | 2 | cattle 139 | 1 | egrets 1.1 | 1 | egrets 4.8 | 4 |
| cattle 70 | 2 | cattle 144 | 2 | egrets 2.1 | 5 | egrets 4.9 | 1 |
| cattle 82 | 1 | cattle 146 | 2 | egrets 2.3 | 5 | egrets 4.10 | 5 |
| cattle 87 | 2 | cattle 151 | 2 | egrets 2.4 | 4 | egrets 4.11 | 4 |
| cattle 101 | 2 | cattle 152 | 2 | egrets 2.5 | 5 | egrets 4.12 | 4 |
| cattle 104 | 1 | cattle 170 | 2 | egrets 2.6 | 3 | egrets 5.1 | 2 |
| cattle 109 | 1 | cattle 175 | 1 | egrets 2.7 | 4 | egrets 5.2 | 3 |
| cattle 115 | 2 | cattle 180 | 2 | egrets 2.8 | 1 | egrets 5.3 | 4 |
| cattle 120 | 2 | cattle 182 | 2 | egrets 4.1 | 5 | egrets 5.4 | 2 |
| cattle 124 | 2 | cattle 204 | 3 | egrets 4.2 | 5 | egrets 5.5 | 4 |
| cattle 127 | 2 | cattle 223 | 1 | egrets 4.3 | 3 | egret 5.6 | 2 |
| cattle 131 | 2 | cattle 231 | 2 | egrets 4.4 | 2 | whisling duck 13 | 3 |
| cattle 135 | 1 | cattle 235 | 1 | egrets 4.5 | 3 | whistling duck 21 | 1 |
a For egrets and whistling ducks, each sample is a pool of five different faecal samples collected on the field
b 218/250 (cattle), 2/28 (egrets) and 22/24 (whistling duck) faecal samples that yielded no cefotaxime resistant isolates are not shown
c Some faecal samples yielded more than one CREC resistant E. coli isolate
d Number of E. coli isolates found resistant to cefotaxime after antimicrobial susceptibility testing of multiple bacterial colonies cultured from each sample on cefotaxime eosine methylene blue agar
All the cefotaxime resistant E. coli (CREC) isolates from the three animal populations with the exception of three egret isolates (LEK 22, LEK 23 and LEK 28) and one cattle isolate (Ct 9) also revealed resistance to at least one other tested antimicrobial (Table 3).
Table 3.
Antimicrobial resistance patterns of the cefotaxime-resistant E. coli isolates
| R-Typea | Egret (n = 87) | Duck (n = 4) | Cattle (n= 53) |
|---|---|---|---|
| CTX | 3 (3.4) | 0 (0.0) | 1 (1.9) |
| CTX, CP | 1 (0.1) | 0 (0.0) | 0 (0.0) |
| CTX, CP, STR, CHL | 0 | 0 (0.0) | 1 (1.9) |
| CTX, CP, TET | 2 (2.3) | 0 (0.0) | 3 (5.7) |
| CTX, CP, TET, CHL | 1 (0.1) | 0 (0.0) | 1 (1.9) |
| CTX, CP, TET, CHL, STR | 2 (2.3) | 0 (0.0) | 0 (0.0) |
| CTX, CP, TET, GEN | 6 (6.9) | 0 (0.0) | 0 (0.0) |
| CTX, CP, TET, GEN, CHL | 0 (0.0) | 0 (0.0) | 2 (3.8) |
| CTX, CP, TET, GEN, CHL, STR | 19 (21.8) | 0 (0.0) | 0 (0.0) |
| CTX, CP, TET, GEN, STR | 3 (3.4) | 1 (25.0) | 0 (0.0) |
| CTX, CP, TET, STR | 4 (4.6) | 0 (0.0) | 0 (0.0) |
| CTX, CP, TET, STR, GEN | 0 (0.0) | 0 (0.0) | 2 (3.8) |
| CTX, CP, TET, STR, GEN, CHL | 0 (0.0) | 0 (0.0) | 9 (17.0) |
| CTX, GEN, CHL | 1 (0.1) | 0 (0.0) | 0 (0.0) |
| CTX, STR | 0 (0.0) | 3 (75.0) | 4 (7.5) |
| CTX, TET | 7 (0.8) | 0 (0.0) | 2 (3.8) |
| CTX, TET, GEN, STR | 2 (2.3) | 0 (0.0) | 0 (0.0) |
| CTX, TET, STR | 36 (41.4) | 0 (0.0) | 28 (52.8) |
aCTX cefotaxime, CP ciprofloxacin, TET tetracycline, GEN gentamicin, CHL chloramphenicol, STR streptomycin
The egret CREC isolates showed highest resistance rate to tetracycline (85/87) followed by streptomycin (69/87) and ciprofloxacin (38/87) (Table 4). The duck CREC isolates showed the highest resistance rate against streptomycin (4/4) followed by equal resistance rate to each of ciprofloxacin, tetracycline and gentamicin (1/4) (Table 4).
Table 4.
Antimicrobial resistance of cefotaxime-resistant E. coli (CREC) isolates of the animals to other antimicrobials
| No. (%) Resistance | |||
|---|---|---|---|
| Antimicrobials | Egrets (n = 87) | Ducks (n = 4) | Cattle (n = 53) |
| Gentamicin | 32 (36.7) | 1 (25.0) | 13 (24.5) |
| Streptomycin | 69 (79.3) | 4 (100) | 46 (86.8) |
| Ciprofloxacin | 38 (43.7) | 1 (25.0) | 17 (32.1) |
| Tetracycline | 85 (97.7) | 1 (25.0) | 47 (88.7) |
| Chloramphenicol | 22 (25.3) | 0 (0.0) | 13 (24.5) |
For the cattle CREC isolates, tetracycline resistance (47/53) was the most commonly detected phenotype. Streptomycin (46/53) and ciprofloxacin resistance (17/53) was also common (Table 4). Multidrug resistance (MDR, resistance to three or more antimicrobial classes) was also observed in many of the CREC isolates: egrets (n = 76), duck (n = 1) and cattle (n = 46) (Table 3). The MDR to cefotaxime, tetracycline and streptomycin (R-type: CTX, TET, STR) predominated amongst both egrets (36/87; 41.4%) and cattle (28/53; 52.8%) isolates. MDR to cefotaxime, ciprofloxacin, tetracycline, gentamicin and streptomycin (CTX, CP, TET, GEN, STR) was found in a single duck isolate (Table 3). All the CREC isolates were susceptible to tested carbapenems.
All the CREC isolates were ESBL-producers; they all harboured ESBL gene blaCTX-M1/15 or blaCTX-M9 with the exception of two egret CREC isolates (LEK 14 and LEK 17). The blaCTX-M1/15 gene was more prevalent than the blaCTX-M9 gene among the CREC isolates: egret, 68 (78.2%) vs. 17 (19.5%); duck, 4 (100%) vs. 0 (0%); and cattle, 52 (98.1%) vs. 1 (1.9%) (Fig. 1), respectively. None of the isolates contained both blaCTX-M1/15 and blaCTX-M9 genes. The blaCTX-M genes were not detected by the microarray-based assays in two cattle egret CREC isolates LEK 14 and LEK 17 but these isolates both harboured the blaCMY gene (Fig. 1). One egret isolate (LEK 70) harboured both blaCTX-M1/15 and blaCMY (Fig. 1). Consensus sequences for blaTEM were found in 56 (64.4%), 4 (100%), 44 (83.0%) of the egret, duck and cattle isolates, respectively. Consensus sequences of the shv gene family were not detected in any of the isolates. The Citrobacter and Salmonella isolates did not contain any ESBL genes. There was full concordance between the cefotaxime resistance and ESBL genotype.
Fig. 1.
Overview of detected antimicrobial resistance and virulence genes in and serotypes of wild birds and cattle ESBL-producing E. coli isolates. *Not detected in the cattle ESBL-producing E. coli isolates; ** Not detected in the wild bird ESBL-producing E. coli isolates. Black boxes indicate gene detection; minus sign under serotype column indicates not detected; a) Egret (Bubulcus ibis) CREC isolates representing the 16 different detected virulence genotypes; b) whistling duck (Dendrocygna viduata) CREC isolates representing the 3 different detected virulence genotypes; c) Cattle CREC isolates representing the 11 different detected virulence genotypes
Several other antimicrobial resistance genes were detected in the CREC isolates. The most prevalent antimicrobial resistance gene in the egret isolates was strB (81.6%) followed by tetA (74.7%). The strB and sul2 genes predominated in the duck isolates with equal prevalence rates (100%); these two genes were also highly prevalent in the cattle isolates (90.6%) (Fig. 1).
Overall, more antimicrobial resistance genes were found in the egret CREC isolates than in the cattle isolates. Eight of the detected non-beta-lactam antimicrobial resistance genes were exclusively found in the egret CREC isolates; armA (aminoglycoside resistance) (n = 1), ble (aminoglycoside) (n = 1), tetG (tetracycline) (n = 1), ermB (macrolide/streptogramin) (n = 1), arr (rifampicin) (n = 10), dfrA19 (trimethoprim) (n = 20), gepA (fluoroquinolone) (n = 4) and floR (chloramphenicol) (n = 12) (Fig. 1).
Class 1 integrons were more prevalent than class 2 integrons in the CREC isolates amongst the isolates recovered from all three animal hosts: egret, 72.4% (n = 63) vs. 1.1% (n = 1); cattle, 77.4% (n = 41) vs. 7.5% (n = 4); whistling duck, 100% (n = 4) vs. 0% (Fig. 1).
Based on the combinations of the ten aminoglycoside genes detected in egret CREC isolates, 13 different genotypes were observed. The most prevalent (14/87) genotype harboured the aac6, aac6Ib and aadA4 genes (Fig. 1). Eight aminoglycoside genes were found in the cattle CREC isolates. The genotype harbouring the aac6, aac6Ib and aadA4 genes was the most prevalent. Only two aminoglycoside genes were found in the whistling duck CREC isolates (Fig. 1).
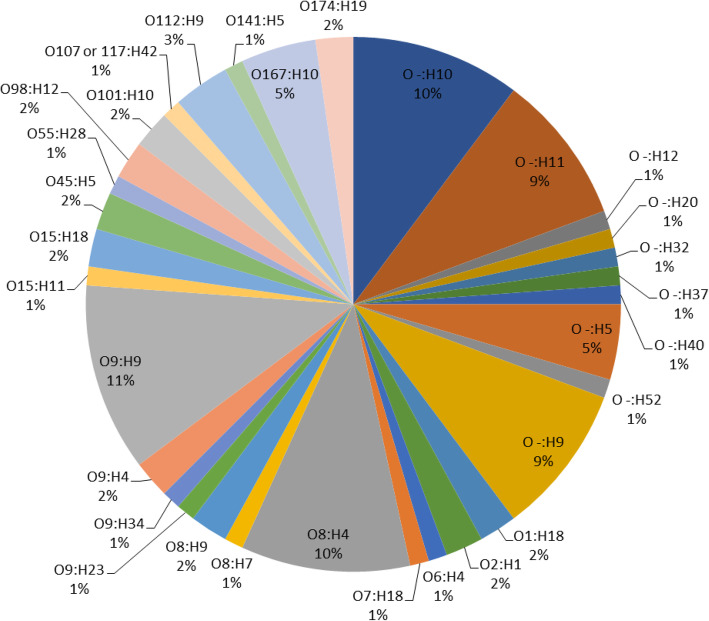
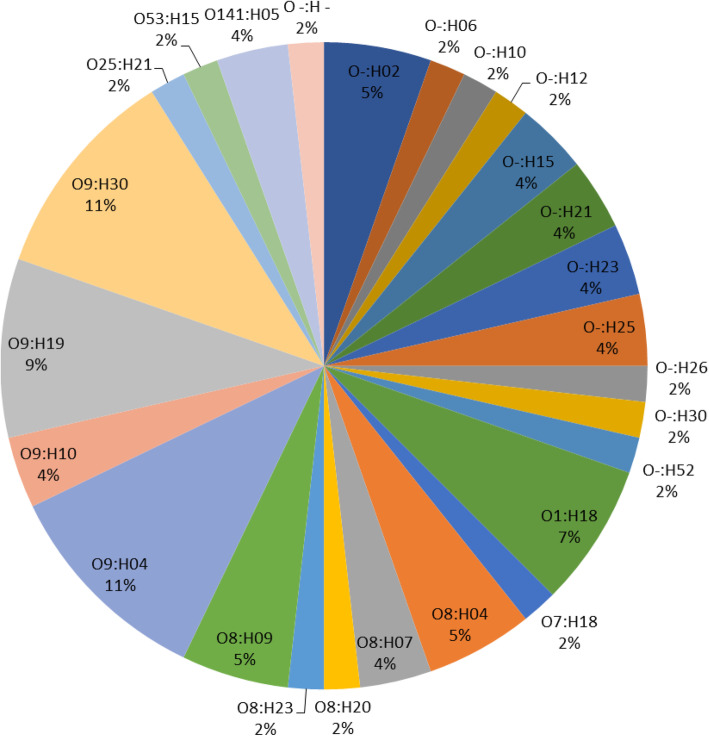
Analysis of O- and H-types of E. coli isolates by SeroGenoTyping AS-1 Kit showed a high diversity of serotypes. A total of 52 (egret), three (duck) and 36 (cattle) CREC isolates could be identified as 22, one and 14 distinct serotypes, respectively (Figs. 1, 2 and 3). The remaining CREC isolates could not be assigned to serotypes as only the H-antigens could be determined. The serotype O9:H9 predominated among the egret CREC isolates (10/87) (Figs. 1, 2). The serotype O9:H30 and O9:H4 were equally prevalent among the cattle CREC isolates (6/53) (Figs. 1 and 3). While three out of the four duck CREC isolates revealed O17/O44/O73/O77/O106:H18 serotype (Fig. 1).
Fig. 2.
Overall distribution of serotypes of cattle egret ESBL-producing E. coli isolates
Fig. 3.
Overall distribution of serotypes of ESBL-producing E. coli isolates of cattle
The O8 and O9 antigens predominated among the egrets (n = 26; 29.9%) and cattle (n = 29; 54.7%) isolates. The E. coli serotypes containing the O8 and O9 antigens included O8:H4, O8:H7, O8:H9, O9:H4, O9:H9, O9:H23, and O9:H34 (egrets); and O8:H20, O8:H4, O8:H23,O8:H9, O8:H7, O9:H4, O9:H19, O9:H30, and O9:H10 (cattle) (Figs. 1, 2 and 3).
Seven distinct serotypes were identified amongst the egret and cattle isolates; O8:H4; O8:H7, O8:H9, O9:H4, O1:H18, O7:H18 and O141:H5. The prevalence rates of serotypes O8:H4 and O9:H4 varied between egret and cattle isolates: serotype O8:H4 was more prevalent in egrets (10% vs. 5%) while serotype O9:H4 was more prevalent in cattle (11% vs. 2%) (Figs. 2 and 3). The duck isolates did not share any serotype with the isolates from other animals. Notably, the same serotype (O8:H4), virulence- and AMR-genotype was identified in one egret (LEK 85) and two cattle isolates (Ct 15 and Ct 16), suggesting clonality (Fig. 1).
Twelve virulence associated genes (VAGs) were detected in the egret CREC isolates. The hemL gene was detected in all the isolates while the other 11 VAGs had a prevalence of equal to or less than 55.2% (Fig. 1). The second most prevalent VAG was iss (increased serum survival) (55.2%) followed by lpfA (28.7%), iroN (19.5%), cma (17.2%) and astA (11.5%). Nine VAGs, however, were detected in the cattle CREC isolates. hemL was also detected in all the cattle isolates while the other VAGs were detected at a prevalence of ≤ 54.7% (Fig. 1). The second most prevalent VAG in the cattle isolates was also iss (54.7%) followed by lpfA (37.7%). Only three VAGs were found in the duck CREC isolates; sepA (three isolates), lpfA (two isolates) and astA (one isolate) (Fig. 1).
Eight VAGs were exclusively detected in the egret isolates: iroN (siderophore receptor) (17/87), cma (15/87), vat (vacuolating autotransporter toxin) (2/87), tsh (temperature-sensitive haemagglutinin) (3/87), mchF (3/84), nleA (1/87) and nfaE (non-fimbrial adhesin) (1/87) (Fig. 1). While perA (1/53), eatA (1/53), cnf1 (cytotoxic necrotizing factor) (2/53), sat1 (secreted autotransporter toxin) (1/53) and iha (adhesin-siderophore receptor) (1/53) were detected only in the cattle (Fig. 1) and sepA (3/4) in the duck isolates (Fig. 1).
Two VAGs were found exclusively in the CREC isolates of egret and cattle: iss (47/87 vs. 27/53) and prfB (2/87 vs. 3/53). While three VAGs (hemL, lpfA and astA) were common to the egret, cattle and whistling duck CREC isolates; only hemL was found in all isolates of the three animal hosts; while lpfA and astA had different prevalence rates in the three animal hosts. The prevalence rates of lpfA were: 25/87 (28.7%) (egret), 20/53 (37.7%) (cattle), 2/4 (50%) (whistling duck), while the prevalence rates of astA were: 10/87 (11.5%) (egret), 4/53 (7.5%) (cattle) and 1/4 (25%) (whistling duck).
A total of 16, 11 and three virulence genotypes were observed in egret, cattle and duck E. coli isolates, respectively (Fig. 1). The VAG genotype harbouring hemL and iss (as in LEK 44 and Ct 44 isolates) predominated the egret and cattle isolates; 25/87 (28.7%) (egret) and 18/53 (34.0%) (cattle), followed by genotype encoding only hemL (as in LEK 18 and Ct 33 isolates) with the prevalence rates of 24/87 (27.6%) (egret) and 14/53 (26.4%) (cattle). The third prevalent VAG genotype in the egrets harboured lpfA, cma, hemL, iroN and iss (as in LEK 13 isolate) with the prevalence rate of 10/87 (11.5%). While, the third most prevalent VAG genotype in the cattle isolates harboured lpfA, hemL and iss (as in Ct 14 isolate) with the prevalence rate of 9/53 (17.0%). The VAG genotype encoding lpfA and hemL (as in LEK 24 and Ct 24 isolates) was shared by the isolates of egret (10/87) (11.5%) and cattle (7/53) (13.2%). While the genotype harbouring astA and hemL (as in LEK 1, Ct 48 and DUD 90 isolates) was detected in the isolates of the three animal hosts with very low prevalence rates; egret (2/87) (2.3%), cattle (1/53) (1.9%) and whistling duck (1/4) (25%).
Varied proportions of CREC isolates of the three animal hosts harboured multiple VAGs (encoding more than 3 VAGs): 26/87 (29.9%) (egret), 14/53(26.4) (cattle) and 2/4 (50%) (egret). The majority (9/10) of the egret isolates having the VAG genotype encoding lpfA, cma, hemL, iroN and iss also showed multidrug resistance to antimicrobials from five antimicrobial classes (cefotaxime, ciprofloxacin, tetracycline, gentamicin, chloramphenicol and streptomycin) compared to only 1/10 isolates showing resistance to only three antimicrobials (cefotaxime, gentamicin and chloramphenicol). Furthermore, all 10 egret isolates having the VAG genotype encoding lpfA, cma, hemL, iroN and iss belonged to serotype O9:H9.The serotype O9:H9 was not common among egret isolates having other VAG genotypes (Fig. 1). Also, the majority (5/8) of cattle isolates having the VAG genotype encoding lpfa, heml and iss belonged to serotype O9:H19 compared with only (3/8) belonging to other serotypes (Fig. 1).
Overall, none of the E. coli isolates could be categorized as one of the molecularly recognised pathogenic strains of E. coli [22, 23] based on the VAGs detected.
Discussion
Our study shows a high carriage rate of ESBL-producing E. coli in cattle egrets and cattle and thus these animals might constitute public health risk. The poor meat hygiene practices in Nigeria could facilitate meat contamination and dissemination during slaughter and transport could lead to a spread to humans through the food chain [25]. The common intermingling of animals with humans in the country could also promote a further spread to humans.
Antimicrobial usage is an important driver of antimicrobial resistance [12]. This factor probably plays a role in the prevalence of antimicrobial resistance in the cattle isolates considering the high and indiscriminate usage of antimicrobials in veterinary and human medicine and animal production in Nigeria [26] as in many African countries [27]. This issue is exacerbated by lack of an antimicrobial control policy and surveillance, off-counter availability of antimicrobials, sub-standard antimicrobials and employment of non-professionals who administer antimicrobials to animals. The high prevalence of MDR isolates is a public health concern as this will complicate treatment of infections caused by these bacteria due to limited therapeutic options. Since wild birds are not directly exposed to antimicrobials, they probably acquired the antimicrobial resistant bacteria through exposure to the environment contaminated with agricultural, animal and human waste.
Wild birds are environmental sentinels for the spread of antimicrobial resistance [18]. The cattle egret and whistling duck are migratory birds and could therefore further spread the bacteria along their transboundary migration routes contaminating water (including those used for irrigational, recreational and drinking purposes), agricultural land and farm produce [28]. They could act as a source of antimicrobial resistant E. coli and resistance determinants to other wildlife [29] through which wild animals including those hunted for meat consumption can also be contaminated. It is noteworthy that the study site for the wild birds is by the bank of a river which supplies raw water to the Water Treatment Plant supplying ‘treated water’ for domestic use of an entire University community. This puts the community at a particular risk due to possibility of contamination of the water by faecal droppings of the birds; the risk is further heightened by the apparent inadequate treatment of the water. Fresh food producing farms were also located close to the study site posing additional risk from the faecal contamination of their products [30]. Additionally, contamination of roof tops by the faecal droppings of the birds could lead to contamination of harvested rain water leading to further transmission through usage of rain water [31], which is common in this environment due to water shortage. Also concerning is the mingling of egrets with cattle at abattoirs, cattle markets and grazing fields through which the birds can spread the bacteria and/or resistance determinants to cattle with the potential of ultimately entering the food chain. A transmission of resistant bacteria from cattle to the egrets is also possible as the birds scavenge abattoir wastes. The indicated phenotypic and genotypic similarity between an egret isolate (LEK 85) and two cattle isolates (Ct 15 and Ct 16) isolates suggests transmission between egrets and cattle reflecting the association of these egrets with cattle. The general practice of slaughtering of animals in open fields in abattoirs make carcasses susceptible to faecal contamination from faecal droppings of egrets and other birds; this calls for biosafety measures in the abattoirs.
The high prevalence of ESBL-producing E. coli in cattle corroborates earlier reports from Africa [13]. An even higher prevalence of 46.6% was reported in Egypt from 210 rectal swab samples collected from dairy cattle [15].
The resistance of these bacteria to other antimicrobials, including ciprofloxacin, would further complicate treatment; however, all the bacteria were susceptible to carbapenems. The susceptibility to carbapenems corresponds to the general low prevalence of carbapenem resistant E. coli in food producing animals in Africa [13, 15, 32].
The predominance of CTX-M15 reported in this study is consistent with the findings in chickens [30] and humans (clinical and asymptomatic) in Nigeria [21, 33] and the global epidemiology of the CTX-M family, including Africa [13]. CTX-M15 is more widespread compared to other CTX-M types which are found in certain locales or host species. The study marks first report of the occurrence of CTX-M9 in Nigeria showing egret as a more prominent reservoir compared to cattle. Report of CTX-M9 in food animals (cattle and chicken) in Africa is not common [15, 34]. It is also noteworthy that E. coli isolates recovered from egrets harboured more antimicrobial resistance genes than those recovered from the other animal species; with exclusive detection of eight antimicrobial resistance genes; armA and ble (aminoglycoside), tetG (tetracycline), ermB (macrolides/streptogramin), arr (rifampicin), dfrA19 (trimethoprim), gepA (fluoroquinolone), floR (chloramphenicol) in the egret isolates. This probably reflects the migratory and scavenging nature of the egrets. The absence of floR gene in the cattle isolates contrasts to the high prevalence of this gene in dairy cattle isolates recovered elsewhere in Africa, such as Egypt [15]; while a floR prevalence rate of 33% was reported in European cattle isolates [8]. The floR gene confers resistance to florfenicol (a fluorinated derivative of chloramphenicol) approved for use in cattle in Europe since 1995 [35].
A majority of the bacteria harboured ≤ 2 VAG. Based on detection of VAGs, none of the E. coli isolates qualified as one of the known major pathotypes of E. coli [22, 23] suggesting their commensal status. Nevertheless, they constitute potential health risk of reservoirs of VAGs. Furthermore, the pathogenic potential of some of these isolates could not be completely ruled out; particularly those which were found to harbour at least one adhesion factor and an important VAG. Those potentially pathogenic E. coli strains included the ones harbouring long polar fimbriae (lpfA) gene which is a potential virulence marker in E. coli [36]. Interestingly many of the isolates encoded the lpfA in addition to at least three other VAGs: 14.9% (egrets) and 7.6% (cattle). Furthermore, the pathogenic potential of the isolates encoding several of the other VAGs cannot be ruled out; for example, the two egret isolates (LEK 14 and LEK 17) encoded six VAGs and the one (LEK 79) encoded five VAGs. The detection of some of the VAGs in certain animal source E. coli isolates probably indicates the pathogenic potential or animal host marker [37].
The findings of this study showed high diversity of the E. coli isolates regarding to their serotype, virulence- and AMR-genotype. However, one egret and two cattle source E. coli isolates had the same O:H serotype and similar VAG and antimicrobial resistance genotype suggesting clonal dissemination between the animals thus calling for control measures. There was a predominance of O9 antigen in the egret and cattle source E. coli isolates, in contrast to prevalence of O111 in dairy cattle in Egypt [15].
There was a high prevalence of class 1 integrons compared to the rare to low prevalence of class 2 integrons in isolates of all animal species which is similar to reports of earlier studies in dairy cattle and asymptomatic humans [15, 38]. However, the prevalence of the class 1 integrons (42/54) in cattle is much higher in this study than 28 out of 114 previously reported in dairy cattle Egypt [15]. Notably also, the prevalence of class 1 integrons in E. coli strains of each animal in this study is more than twice the 31% earlier reported for E. coli strains in asymptomatic humans in Nigeria [38] thus suggesting animals as more important reservoirs of integrons in the country. Integrons play an important role in the development of multidrug resistance and dissemination of antimicrobial resistance in bacteria [39].
Conclusion
Cattle egrets (Bubulcus ibis), whistling ducks (Dendrocygna viduata) and cattle constitute important reservoirs of highly diverse extended spectrum β-lactamase producing E. coli with potentials of inter-species transmission, widespread and virulence. The possible public health risks include contamination of the environment by the faeces of the migratory wild birds and cattle. The antimicrobial resistant bacteria could also spread through the food chain if beef meat is contaminated during slaughtering and butchering of cattle; through use of livestock faeces as manure; additionally, the birds can contaminate farm lands and agricultural products and water bodies with their faecal droppings. The findings of this study indicate the need for implementation of recommended guidelines for co-management of public health and habitat conservation; necessary regional specific guidelines can also be researched. Furthermore, meat hygiene should be adequately enforced at abattoirs to prevent contamination of meat; biosecurity measures should also be put in place to prevent wild bird intrusion into meat processing areas. There is need for formulation and enforcement of policies to regulate use of antimicrobials in the country; antimicrobial surveillance programme is also necessary. Public health education about health implications of indiscriminate use of antimicrobials is important.
Methods
Study sites
Samples were collected between January and July, 2016. Wild bird samples were taken on the banks of the river Awba and from cattle at the Bodija abattoir in Ibadan, Nigeria.
The River Awba is a second order river catchment that drains University of Ibadan, Ibadan, Nigeria. The river basin is situated between latitudes 7 o 25′58″ and 7 o26’42″ and longitudes 3o 53′21″ and 3o 54′26″ East of Greenwich Meridian. The drainage area is 2.08 km2, its drainage density is 1.93 km/km2 [40]. The river drains some residential areas (staff quarters and student hostels), department of fisheries and aquaculture and academic departments in the Faculties of Science and Technology; after which a dam, called Awba Dam, is located. The dam supplements raw water supply to the University water treatment plant which processes and supplies water for domestic use and drinking of the University community. Fishing activity and fish breeding also take place in the dam. The trees on one of the dam banks serve as roosting sites for cattle egrets (Bubulcus ibis) during every dry season (early October to mid-May). The banks are also colonised by White-faced whistling ducks (Dendrocygna viduata) during the same period.
The cattle egrets sleep on treetops overnight. During dry season, each morning (around 7 a.m.), the birds disperse in groups in different directions into the city to look for food, and they start to return just before it gets dark (around 6.30 p.m.). When they arrive in the evening, they first land on the banks to drink water and then fly to perch on top of the trees to sleep till next morning; this is the daily routine of the egrets until the end of the dry season when they finally migrate away. Under the trees, the ground is usually littered with the faeces of the egrets.
Whistling ducks do not perch on the trees but swim in the Awba reservoir and restrict themselves only to the bank without trees when not swimming; the banks are littered with the faeces of the whistling ducks. Bodija abattoir is the main abattoir in Ibadan where cattle and other animals apart from poultry are slaughtered for meat consumption.
Collection of faecal samples of birds and cattle
The Awba Dam was visited four times between January and March in 2016 (at intervals of 3 weeks) and in this period pooled faecal samples of egrets (n = 28) and whistling ducks (n = 24) were collected separately. To collect faecal samples of egrets, sterile aluminium foil was randomly spread under the trees in the evening and left it overnight to collect faecal droppings. Early in the morning at the next day, fresh faecal samples were collected into a sterile stool bottle. Faecal samples of ducks were randomly collected from the bank of the Awba Dam. Colour and consistency of faeces were used to discriminate between wild duck and egret samples to avoid a mixture of both. After collection, five randomly collected samples for each bird species were pooled to one sample. All faecal bird samples were transported to the microbiology laboratory within 2 h.
Cattle faecal samples (n = 250) were collected between April and July 2016 at the slaughter of the Bodija Abattoir. One sample per cattle was collected by the veterinarians on duty directly from the rectum into a sterile stool bottle using sterile gloves. The faecal samples were transported to the laboratory within 2 h.
Culturing of faecal samples
Each faecal sample was thoroughly mixed inside the bottle using a sterile cotton swab stick and streaked onto 8 μg/mL cefotaxime-eosin-methylene blue agar (CEMB) (Lab M, Lancashire, UK). Afterwards, the plates were incubated aerobically at 37 °C for 18 h. At least two bacterial colonies were subcultured (depending on the number of colonies on the primary plate) on fresh plates of CEMB agar and incubated as above. Colonies were then inoculated onto a nutrient agar slope (Lab M, Lancashire, UK) and incubated as above. All 149 isolates were subjected to biochemical tests [41]. E. coli was identified by Gram staining, oxidase and citrate assay (negative) and indole, glucose, lactose assays (positive). E. coli isolates were further analysed genotypically by a microarray-based assay using the E. coli PanType AS-2 Kit [Abbott (Alere Technologies GmbH), Jena, Germany].
Antimicrobial susceptibility testing
Susceptibility tests of all 149 bacterial isolates yielded 144 ones that where resistant to cefotaxime. These 144 isolates were further tested for susceptibility to other antimicrobials using disk diffusion assay on Müller-Hinton agar (Merck, Hamburg, Germany) following the guidelines of Clinical and Laboratory Standards Institute using E. coli ATCC 25922 as a quality control strain [42]. Nine different antimicrobial disks (Oxoid, Basingstoke, Hampshire, United Kingdom) were tested; ampicillin (10 μg), cefotaxime (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), streptomycin (10 μg), imipenem (10 μg), ertapenem (10 μg) and tetracycline (30 μg). Diameters of zones of inhibition were measured with a ruler and interpreted according to the CLSI guidelines [43]. Only cefotaxime resistant isolates were subjected to further analysis. ESBL production was confirmed by the double disc synergy test as described by the Clinical and Laboratory Standards Institute.
Genomic DNA extraction
Genomic DNA from the bacterial isolates was extracted using the DNeasy Blood and Tissue kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s instructions. When necessary, DNA was concentrated to at least 100 ng/μL using a SpeedVac centrifuge (Eppendorf, Hamburg, Germany) at room temperature with 1400 rpm for 30 min. Aliquots of 5 μL of genomic DNA were used directly for biotin-labelling and subsequent hybridization.
GenoSeroTyping and antimicrobial resistance genotyping
The genoserotype of E. coli isolates was determined using the SeroGenoTyping AS-1 Kit (Abbott, Jena, Germany); while the AMR genotype was detected by both the CarbDetect AS-2 (for non-E. coli isolates) and the E. coli PanType AS-2 Kit. All kits were used according to manufacturer’s instructions. The Result Collector 2.0 (Abbott, Jena, Germany) automatically summarized the results.
An antibiotic resistance genotype was defined as a group of known genes conferring resistance to a family of antibiotics (e.g., the genotype “blaCTX-M1/15” confers resistance to 3GC) (Fig. 1).
Multiplex Labelling, hybridization, and data analysis
Extracted DNA was labelled by primer extension amplification using E. coli SeroGenoTyping AS-1 [44], CarbDetect AS-2 [45] or E. coli PanType AS-2 [46] kits according to manufacturer’s instructions. The multiplex labelling, hybridization and data analysis were described in very detail by Braun et al. 2014 [47].
Acknowledgments
Jimoh Abiola of the Department of Botany, University of Ibadan is appreciated for assistance with sample collection.
Abbreviations
- ESBLs
Extended-spectrum beta-lactamases
- AMR
Antimicrobial resistance
- 3GC
Third generation cephalosporins
- VAGs
Virulence associated genes
- CEMB
Cefotaxime-eosine methylene blue agar
- CREC
Cefotaxime resistant E. coli
- MDR
Multidrug resistant
Authors’ contributions
Conceived and designed the experiments: KF, SB and RE. Performed the experiments: KF, IE and SB. Analysed the data: KF, IE, SM, SB and RE. Wrote the draft: KF. Review and edit manuscript: KF, IE, SM, SB, and RE. All authors have read and approved the manuscript.
Funding
The author(s) received no specific funding for this work. KF was granted access to available facilities and reagents at the institution coupled with space and time to carry out the experiments.
Availability of data and materials
The complete raw data sets used during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by Department of Wildlife and Ecotourism, University of Ibadan and Ibadan-North Local Government Authority, Ibadan which granted permission to collect the specimens. Clearance by an ethics committee was not necessary. The specimens were obtained by non-invasive procedures as described in the materials and methods (i.e., rectal swabbing of cattle at abattoir and collection of bird faecal droppings from the environment without animal contact). No animal experiments were carried out for this study. Verbal informed consent of personnels, cattle owners and butchers (at the abattoir) was obtained to collect the faecal samples.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coque TM, Baquero F, Canton R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008;13(47) Epub 2008/11/22. PubMed PMID: 19021958. [PubMed]
- 2.Cosgrove SE. Evidence that prevention makes cents: costs of catheter-associated bloodstream infections in the intensive care unit. Crit Care Med. 2006;34(8):2243–2244. doi: 10.1097/01.CCM.0000229637.32517.90. [DOI] [PubMed] [Google Scholar]
- 3.Tumbarello M, Spanu T, Di Bidino R, Marchetti M, Ruggeri M, Trecarichi EM, et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother. 2010;54(10):4085–4091. doi: 10.1128/AAC.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64(Suppl 1):i3–10. doi: 10.1093/jac/dkp256. [DOI] [PubMed] [Google Scholar]
- 5.Livermore DM, Hawkey PM. CTX-M: changing the face of ESBLs in the UK. J Antimicrob Chemother. 2005;56(3):451–454. doi: 10.1093/jac/dki239. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Bano J, Gutierrez-Gutierrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-beta-lactamase-, ampC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2) Epub 2018/02/16. PubMed PMID: 29444952; PubMed Central PMCID: PMC5967687. 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed]
- 7.Stokes MO, Cottell JL, Piddock LJ, Wu G, Wootton M, Mevius DJ, et al. Detection and characterization of pCT-like plasmid vectors for blaCTX-M-14 in Escherichia coli isolates from humans, turkeys and cattle in England and Wales. J Antimicrob Chemother. 2012;67(7):1639–1644. doi: 10.1093/jac/dks126. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Day MJ, Mafura MT, Nunez-Garcia J, Fenner JJ, Sharma M, et al. Comparative analysis of ESBL-positive Escherichia coli isolates from animals and humans from the UK, the Netherlands and Germany. PLoS One. 2013;8(9):e75392. doi: 10.1371/journal.pone.0075392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canton R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9(5):466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M beta-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72(8):2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 11.Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5(5):449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 12.Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother. 2014;69(3):827–834. doi: 10.1093/jac/dkt443. [DOI] [PubMed] [Google Scholar]
- 13.Alonso CA, Zarazaga M, Ben Sallem R, Jouini A, Ben Slama K, Torres C. Antibiotic resistance in Escherichia coli in husbandry animals: the African perspective. Lett Appl Microbiol. 2017;64(5):318–334. doi: 10.1111/lam.12724. [DOI] [PubMed] [Google Scholar]
- 14.Brandt C, Braun SD, Stein C, Slickers P, Ehricht R, Pletz MW, et al. In silico serine beta-lactamases analysis reveals a huge potential resistome in environmental and pathogenic species. Sci Rep. 2017;7:43232. doi: 10.1038/srep43232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun SD, Ahmed MF, El-Adawy H, Hotzel H, Engelmann I, Weiss D, et al. Surveillance of extended-Spectrum Beta-lactamase-producing Escherichia coli in dairy cattle farms in the Nile Delta. Egypt Front Microbiol. 2016;7:1020. doi: 10.3389/fmicb.2016.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson DGJ, Andremont A, Bengtsson-Palme J, Brandt KK, de Roda Husman AM, Fagerstedt P, et al. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ Int. 2018;117:132–138. doi: 10.1016/j.envint.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 17.Overdevest I, Willemsen I, Rijnsburger M, Eustace A, Xu L, Hawkey P, et al. Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans. The Netherlands Emerg Infect Dis. 2011;17(7):1216–1222. doi: 10.3201/eid1707.110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Ma ZB, Zeng ZL, Yang XW, Huang Y, Liu JH. The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zool Res. 2017;38(2):55–80. doi: 10.24272/j.issn.2095-8137.2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EPoBH B. Scientific opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J. 2011;9:2322. doi: 10.2903/j.efsa.2011.2322. [DOI] [Google Scholar]
- 20.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect. 2011;17(6):873–880. doi: 10.1111/j.1469-0691.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 21.Fortini D, Fashae K, Villa L, Feudi C, Garcia-Fernandez A, Carattoli A. A novel plasmid carrying blaCTX-M-15 identified in commensal Escherichia coli from healthy pregnant women in Ibadan, Nigeria. J Glob Antimicrob Resist. 2015;3(1):9–12. doi: 10.1016/j.jgar.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JR, Murray AC, Gajewski A, Sullivan M, Snippes P, Kuskowski MA, et al. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob Agents Chemother. 2003;47(7):2161–2168. doi: 10.1128/AAC.47.7.2161-2168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak K, Fahr J, Weber N, Lubke-Becker A, Semmler T, Weiss S, et al. Highly diverse and antimicrobial susceptible Escherichia coli display a naive bacterial population in fruit bats from the republic of Congo. PLoS One. 2017;12(7):e0178146. doi: 10.1371/journal.pone.0178146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachiri T, Bakour S, Ladjouzi R, Thongpan L, Rolain JM, Touati A. High rates of CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae in wild boars and Barbary macaques in Algeria. J Glob Antimicrob Resist. 2017;8:35–40. doi: 10.1016/j.jgar.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Kluytmans JA, Overdevest IT, Willemsen I, Kluytmans-van den Bergh MF, van der Zwaluw K. Heck M, et al. extended-spectrum beta-lactamase-producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis. 2013;56(4):478–487. doi: 10.1093/cid/cis929. [DOI] [PubMed] [Google Scholar]
- 26.Adesokan HK, Akanbi IO, Akanbi IM, Obaweda RA. Pattern of antimicrobial usage in livestock animals in south-western Nigeria: The need for alternative plans. Onderstepoort J Vet Res. 2015;82(1):816. doi: 10.4102/ojvr.v82i1.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimera ZI, Mshana SE, Rweyemamu MM, Mboera LEG, Matee MIN. Antimicrobial use and resistance in food-producing animals and the environment: an African perspective. Antimicrob Resist Infect Control. 2020;9(1):37. doi: 10.1186/s13756-020-0697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greig J, Rajic A, Young I, Mascarenhas M, Waddell L, LeJeune J. A scoping review of the role of wildlife in the transmission of bacterial pathogens and antimicrobial resistance to the food chain. Zoonoses Public Health. 2015;62(4):269–284. doi: 10.1111/zph.12147. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler E, Hong PY, Bedon LC, Mackie RI. Carriage of antibiotic-resistant enteric bacteria varies among sites in Galapagos reptiles. J Wildl Dis. 2012;48(1):56–67. doi: 10.7589/0090-3558-48.1.56. [DOI] [PubMed] [Google Scholar]
- 30.Jay MT, Cooley M, Carychao D, Wiscomb GW, Sweitzer RA, Crawford-Miksza L, et al. Escherichia coli O157:H7 in feral swine near spinach fields and cattle, Central California coast. Emerg Infect Dis. 2007;13(12):1908–1911. doi: 10.3201/eid1312.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayebare SR, Wilson LR, Carpenter DO, Dziewulski DM, Kannan K. A review of potable water accessibility and sustainability issues in developing countries - case study of Uganda. Rev Environ Health. 2014;29(4):363–378. doi: 10.1515/reveh-2013-0019. [DOI] [PubMed] [Google Scholar]
- 32.Ojo OE, Schwarz S, Michael GB. Detection and characterization of extended-spectrum beta-lactamase-producing Escherichia coli from chicken production chains in Nigeria. Vet Microbiol. 2016;194:62–68. doi: 10.1016/j.vetmic.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Aibinu I, Odugbemi T, Koenig W, Ghebremedhin B. Sequence type ST131 and ST10 complex (ST617) predominant among CTX-M-15-producing Escherichia coli isolates from Nigeria. Clin Microbiol Infect. 2012;18(3):49–51. doi: 10.1111/j.1469-0691.2011.03730.x. [DOI] [PubMed] [Google Scholar]
- 34.Grami R, Mansour W, Dahmen S, Mehri W, Haenni M, Aouni M, et al. The blaCTX-M-1 IncI1/ST3 plasmid is dominant in chickens and pets in Tunisia. J Antimicrob Chemother. 2013;68(12):2950–2952. doi: 10.1093/jac/dkt258. [DOI] [PubMed] [Google Scholar]
- 35.Cloeckaert A, Baucheron S, Flaujac G, Schwarz S, Kehrenberg C, Martel JL, et al. Plasmid-mediated florfenicol resistance encoded by the floR gene in Escherichia coli isolated from cattle. Antimicrob Agents Chemother. 2000;44(10):2858–2860. doi: 10.1128/AAC.44.10.2858-2860.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres AG, Blanco M, Valenzuela P, Slater TM, Patel SD, Dahbi G, et al. Genes related to long polar fimbriae of pathogenic Escherichia coli strains as reliable markers to identify virulent isolates. J Clin Microbiol. 2009;47(8):2442–2451. doi: 10.1128/JCM.00566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dho-Moulin M, Fairbrother JM. Avian pathogenic Escherichia coli (APEC) Vet Res. 1999;30(2–3):299–316. [PubMed] [Google Scholar]
- 38.Odetoyin BW, Labar AS, Lamikanra A, Aboderin AO, Okeke IN. Classes 1 and 2 integrons in faecal Escherichia coli strains isolated from mother-child pairs in Nigeria. PLoS One. 2017;12(8):e0183383. doi: 10.1371/journal.pone.0183383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carattoli A. Importance of integrons in the diffusion of resistance. Vet Res. 2001;32(3–4):243–259. doi: 10.1051/vetres:2001122. [DOI] [PubMed] [Google Scholar]
- 40.Adediji A, Fashae OA. Sediment dynamics in a small, 2nd order urban river awba catchment, Ibadan, Nigeria. Journal of Environl Geog. 2014;7(1–2):23–28. [Google Scholar]
- 41.Cowan ST. Cowan and steel’s manual for the identification of medical bacteria. 2. Cambridge: Cambridge University Press; 1974. [Google Scholar]
- 42.CaLSI CLSI. Performance standards for antimicrobial disk susceptibility tests; approved standard. 12 2015. [Google Scholar]
- 43.CaLSI CLSI. Performance standards for antimicrobial disk susceptibility testing. 25 2015. [Google Scholar]
- 44.Ballmer K, Korczak BM, Kuhnert P, Slickers P, Ehricht R, Hachler H. Fast DNA serotyping of Escherichia coli by use of an oligonucleotide microarray. J Clin Microbiol. 2007;45(2):370–379. doi: 10.1128/JCM.01361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun SD, Jamil B, Syed MA, Abbasi SA, Weiss D, Slickers P, et al. Prevalence of carbapenemase-producing organisms at the kidney Center of Rawalpindi (Pakistan) and evaluation of an advanced molecular microarray-based carbapenemase assay. Fut Microbiol. 2018. Epub 2018/06/26. PubMed PMID: 29938540. 10.2217/fmb-2018-0082. [DOI] [PubMed]
- 46.Geue L, Schares S, Mintel B, Conraths FJ, Muller E, Ehricht R. Rapid microarray-based genotyping of enterohemorrhagic Escherichia coli serotype O156:H25/H−/Hnt isolates from cattle and clonal relationship analysis. Appl Environ Microbiol. 2010;76(16):5510–5519. doi: 10.1128/AEM.00743-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun SD, Monecke S, Thurmer A, Ruppelt A, Makarewicz O, Pletz M, et al. Rapid identification of carbapenemase genes in gram-negative bacteria with an oligonucleotide microarray-based assay. PLoS One. 2014;9(7):e102232. doi: 10.1371/journal.pone.0102232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The complete raw data sets used during the current study are available from the corresponding author on reasonable request.