SUMMARY
Tactile stimuli are integrated and processed by neuronal circuits in the deep dorsal horn of the spinal cord. Several spinal interneuron populations have been implicated in tactile information processing. However, dorsal horn projection neurons that contribute to the postsynaptic dorsal column (PSDC) pathway transmitting tactile information to the brain are poorly characterized. Here, we show that spinal neurons marked by the expression of Zic2creER mediate light touch sensitivity and textural discrimination. A subset of Zic2creER neurons are PSDC neurons that project to brainstem dorsal column nuclei, and chemogenetic activation of Zic2 PSDC neurons increases sensitivity to light touch stimuli. Zic2 neurons receive direct input from the cortex and brainstem motor nuclei and are required for corrective motor movements. These results suggest that Zic2 neurons integrate sensory input from cutaneous afferents with descending signals from the brain to promote corrective movements and transmit processed touch information back to the brain.
In Brief
The dorsal column projection transmits touch information from the spinal cord to the brain. Paixão et al. describe that Zic2 neurons contribute to this projection. Zic2 neurons also integrate sensory feedback with information from the brain to generate appropriate movements.
Graphical Abstract
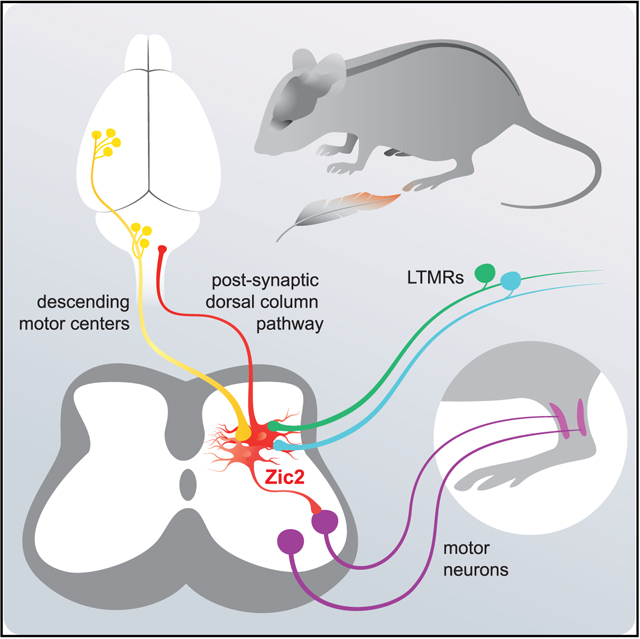
INTRODUCTION
The dorsal horn of the spinal cord is a key waystation for processing and transmitting somatosensory information about the internal and external environment. The exteroceptive arm of the somatosensory system, which comprises specialized skin receptors and their associated sensory afferent subtypes, conveys information about touch, temperature, pruritic (itch), and painful (noxious) stimuli. This information is transmitted by specialized peripheral afferents that relay different sensory modalities and project to different dorsal horn laminae. Nociceptive and thermal information is transmitted to the superficial laminae I/II, while innocuous touch transmitted by low threshold mechanoreceptor (LTMR) afferents project to the LTMR recipient zone (LTMR-RZ) where they synapse with interneurons (INs) in laminae IIi–V (Abraira and Ginty, 2013; Koch et al., 2018; Moehring et al., 2018).
The functional analysis of INs within the LTMR-RZ is still in its infancy. Recent analysis of 11 LTMR-RZ IN populations revealed that each population receives input from at least two LTMR afferent subtypes, as well as other dorsal horn INs and corticospinal neurons, suggesting roles in integrating multiple touch modalities with descending input from higher brain centers (Abraira et al., 2017). Excitatory INs in the LTMR-RZ relaying innocuous touch contribute to the dynamic control of movement, whereas inhibitory IN populations gate and prevent innocuous stimuli from activating the nociceptive pathway (Koch et al., 2018). Excitatory INs in laminae IIi–III expressing the nuclear orphan receptor RORα transmit light touch information and integrate cutaneous sensory feedback with descending motor commands to generate corrective motor movements (Bourane et al., 2015). RORα INs partially overlap with excitatory cholecystokinin (CCK) INs, which are broadly distributed throughout the dorsal horn. Chronic silencing of CCK INs impairs tactile discrimination without affecting responses to noxious heat, and similar impairments in tactile discrimination were described in mice after chronic silencing of the RORβ IN subpopulation (Abraira et al., 2017; Koch et al., 2017). Excitatory and inhibitory INs within the LTMR-RZ also receive noxious mechanical input and serve to modulate nociceptive information before it is relayed to supraspinal sites. Several genetically identified subpopulations of INs, including those that express CCK, have also been implicated in the development of mechanical allodynia, where touch is perceived as painful (Cheng et al., 2017; Cui et al., 2016; Duan et al., 2014; Liu et al., 2018; Peirs et al., 2015; Petitjean et al., 2015).
Most LTMRs also extend ascending axon branches, which form the so-called “direct dorsal column pathway,” a “labeled line” that transmits largely unprocessed touch information to the CNS. Tactile information is also processed and integrated within the dorsal horn LTMR-RZ by local circuit INs, with tactile information then relayed to the brain by projection neurons of the postsynaptic dorsal column (PSDC) and anterolateral tract. Although representing fewer than 2% of neurons in this region, these projection neurons are thought to play important roles in interpreting and relaying tactile information to the brain (Abraira and Ginty, 2013; Dobry and Casey, 1972). PSDC axons travel through the dorsal columns, intermingled with those of the direct pathway, to synapse with neurons in the brainstem dorsal column nuclei (DCN). However, unlike local circuit dorsal horn INs, the molecular identity of PSDC neurons has not yet been determined. We previously characterized deep lamina excitatory neurons marked by the expression of the transcription factor Zic2 and the guidance receptor EphA4 during development (Paixão et al., 2013). We proposed that a subpopulation of Zic2 neurons formed an ascending projection in the dorsal column and might be considered to be PSDC neurons. Zic2 neurons were surrounded by Ret+ Aβ-mechanosensory afferent terminals, raising the possibility that these neurons relay discriminative touch sensation. Functional characterization of these neurons in the adult was not possible, because Zic2 expression was rapidly extinguished after birth.
Here, we have functionally characterized the Zic2 spinal neuron population. Ablation of Zic2 neurons led to reduced sensitivity to light touch and tactile discrimination. Conversely, pharmacogenetic activation of Zic2 spinal neurons increased sensitivity to tactile stimulations that promoted conditioned place aversion, indicating the negative valence of such activation. Mice lacking Zic2 spinal neurons also showed impaired fine motor control, suggesting a role for Zic2 neurons in the integration of cutaneous sensory input with descending motor signals. A subpopulation of Zic2 neurons projects to the DCN, and the selective activation of projecting Zic2 neurons leads to hypersensitivity to light touch, revealing that Zic2 projection neurons play an important role in transmitting innocuous tactile information to the somatosensory cortex by the DCN.
RESULTS
Gaining Genetic Access to Zic2 Neurons
To characterize and manipulate Zic2 neurons, we generated a bacterial artificial chromosome (BAC) transgenic mouse by using the same strategy previously used for generating the Zic2::eGFP mouse (Escalante et al., 2013; Paixão et al., 2013). EGFP was substituted by the tamoxifen-inducible version of Cre recombinase (CreER). Two transgenic founder lines were selected, namely, Zic2creER and Zic2creERbroad (Figures 1A and S1A). Because Zic2 expression is highly dynamic and downregulated after birth (Escalante et al., 2013), we characterized the pattern of Zic2cre-dependent recombination by comparing the signal of the Ai9 Cre-dependent tdTomato reporter (Madisen et al., 2010) to endogenous Zic2 immunoreactivity (IR) at embryonic day 16.5 (E16.5), 2 days after Cre induction. The Zic2creER line showed a good overlap with Zic2 IR, especially in the medial spinal cord region in laminae III-V (Figures 1B and S1C). The Zic2creERbroad line showed a similar pattern of expression and overlapped with Zic2 IR in a larger number of neurons, with an additional population of more lateral dorsal horn neurons that lacked endogenous Zic2 IR (Figures S1B and S1C). Importantly, tdTomato expression in Zic2creER mice faithfully reported the absence of endogenous Zic2 IR in sensory neurons in the dorsal root ganglia (DRGs), whereas sporadic tdTomato cells were observed in the Zic2creERbroad line (Figures 1A and S1A). For these reasons, we mainly used the Zic2creER line and the Zic2creERbroad line to confirm our results.
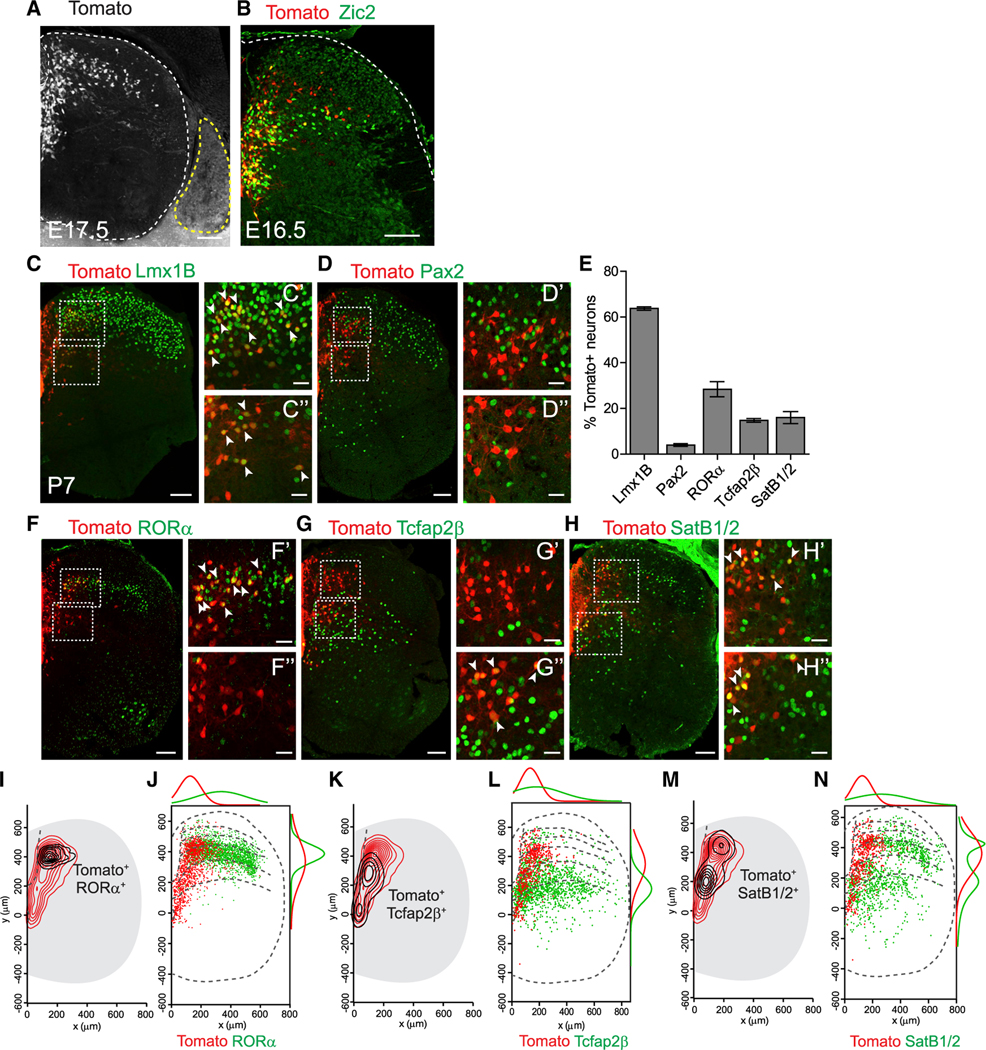
Figure 1. Characterization of Zic2creER Neurons.
(A and B) Spinal cord (SC) transverse sections of Zic2creER::Ai9 (A) showing tdTomato expression (red) and Zic2 IR (green) (B). Double-positive cells appear yellow. Note that DRGs (yellow stippled line) do not express tdTomato.
(C, D, and F–H) Immunostainings of Zic2creER::Ai9 lumbar SC with indicated markers (green): Lmx1B (C), Pax2 (D), RORα (F), Tcfap2β (G), SatB1/2 (H), and tdTomato expression (red). Boxed areas indicate higher magnification regions from laminae III–IV (C’, D’, F’, G’, and H’) and lamina V (C”, D”, F”, G”, and H”). Double-positive cells indicated with arrowheads.
(E) Percentage of Zic2creER-tdTomato cells expressing indicated markers (n = 3/4 sections from 3 mice each). Data represented as mean ± SEM.
(I, K, and M) Contour density plots of Zic2creER cells (red lines) and double-positive cells for indicated markers (black lines): RORα (I), Tcfap2β (K), and SatB1/2 (M) on lumbar SC.
(J, L, and N) Comparison of spatial distributions of individual Zic2creER cells (red) and indicated markers (green): RORα (J), Tcfap2β (L), SatB1/2 (N), and mediolateral (top) and dorso-ventral (right) frequency distributions represented as a non-linear regression.
Scale bars, 100 μm (A–H), 30 μm (C’–H”).
See Figure S1.
In postnatal spinal cords, the majority of Zic2creER;tdTomato neurons (63.8% ± 0.8%) expressed the glutamatergic dorsal horn marker Lmx1B (Cheng et al., 2004; Ding et al., 2004) (Figures 1C and 1E), whereas very few (4% ± 0.6%) expressed the inhibitory marker Pax2 (Glasgow et al., 2005) (Figures 1D and 1E). A significant proportion (28.4% ± 3.3%) expressed RORα, a marker of excitatory INs important for transmitting light touch (Bourane et al., 2015), whereas Zic2creER;tdTomato constitute a smaller proportion of RORα neurons (16.9% ± 0.9%). The population showing overlap was located in the most medial region of laminae III–IV, with the Zic2+/RORα neurons extending to more medial and deeper lamina V and the Zic2 /RORα+ INs to more lateral parts of laminae III–IV (Figures 1E, 1F,1I, and 1J). Zic2 neurons also co-localized with the lamina V markers Tcfap2β (14.8% ± 0.8%) and SatB1/2 (16% ± 2.6%) that label a population of motor synergy encoder INs (Hilde et al., 2016; Levine et al., 2014) (Figures 1E, 1G, 1H, and 1K–1N). Although the Zic2+/ Tcfap2β+ subpopulation was largely restricted to lamina V, the Zic2+/SatB1/2+ subpopulation ranged from laminae III to V.
Ablation of Zic2 Spinal Neurons Reduces Light Touch Sensitivity
To functionally characterize Zic2 spinal neurons, we specifically ablated Zic2 neurons in the adult spinal cord by using intersectional genetics. This was achieved by combining the Flp- and Cre-dependent Tauds-DTR allele expressing the diphtheria toxin receptor (DTR) with Zic2creER and the Cdx2FlpO transgenes. Expression of Cdx2FlpO (optimized Flp) was confined to the caudal region of the mouse embryo, from mid-cervical levels in the spinal cord (Britz et al., 2015). We induced Cre expression in Zic2 spinal neurons at E14.5 and caused ablation of Zic2 neurons at 6 weeks postnatally by application of diphtheria toxin (DTx) (Figures 2A and 2B). Based on the Ai14 Cre-dependent tdTomato reporter allele (Madisen et al., 2010), 60% of Zic2 neurons were ablated in the lumbar spinal cord (Figures 2C and 2D). DTx treatment seemed to equally affect all Zic2 neurons because the spatial distribution of the remaining non-ablated Zic2 neurons was similar to the distribution of the intact population (Figures S2A–S2D).
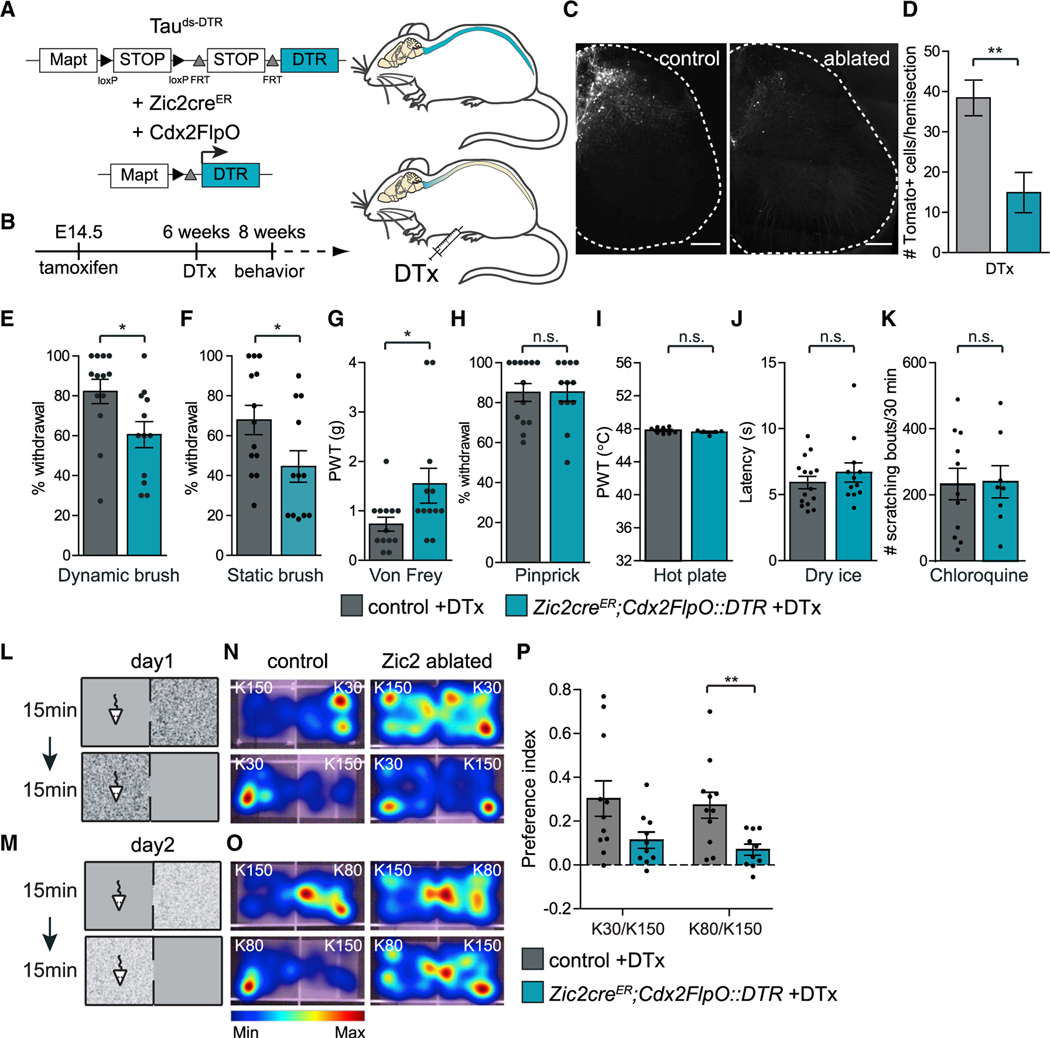
Figure 2. Ablating Spinal Zic2 Neurons Impairs Light Touch Sensitivity.
(A) Experimental strategy to ablate Zic2creER neurons in the SC. Mapt promoter; FRT and loxP, recognition sites for Flp and Cre recombinases, respectively; DTR, diphtheria toxin receptor; DTx, diphtheria toxin.
(B) Experimental paradigm time line.
(C) Adult transverse lumbar SC sections of Zic2creER::DTR;Ai14 (control) and Zic2creER;Cdx2FlpO::DTR;Ai14 (ablated) treated with DTx. Scale bars, 200 μm.
(D) Quantification of ablation efficiency (control: 38.4 ± 4.4 cells, n = 3/4 sections, 5 mice; ablated: 14.9 ± 5 cells, n = 4 sections, 7 mice; two-tailed Student’s unpaired t test, t(10) = 3.3; **p = 0.008).
(E and F) Ablated mice show a significant decrease in paw withdrawal to dynamic brush (E) (control: 82.2% ± 6.1%, n = 13; ablated: 60.5% ± 6.5%, n = 12; two-tailed Student’s unpaired t test; t(23) = 2.4; *p = 0.02) and static brush (F) (control: 67.9% ± 7.3%, n = 13; ablated: 44.6% ± 7.9%, n = 12; two-tailed Student’s unpaired t test; t(23) = 2.2; *p = 0.04).
(G) Ablated mice show a significant increase in paw withdrawal thresholds (PWTs) to von Frey (control: 0.7 ± 0.1 g, n = 13; ablated: 1.6 ± 0.4 g, n = 12; two-tailed Student’s unpaired t test, t(23) = 2.2, *p = 0.04).
(H) No detectable changes in response to pinprick (control: 85.1% ± 4.4%, n = 13; ablated: 85.3% ± 4.5%, n = 12; two-tailed Student’s unpaired t test, t(23) = 0.03, p = 1).
(I) Heat sensitivity measured by PWTs in the dynamic hot plate was not altered in ablated mice (control: 47.8° C ± 0.1° C, n = 8; ablated: 47.6° C ± 0.1° C, n = 5; two-tailed Student’s unpaired t test, t(11) = 1.1, p = 0.3).
(J) Cold sensitivity measured by the latency to withdraw the paw in response to dry-ice stimulation was not altered in ablated mice (control: 5.9 ± 0.5 s, n = 15; ablated: 6.7 ± 0.7 s, n = 12; two-tailed Student’s unpaired t test; t(25) = 0.9, p = 0.4).
(K) Scratch response to chloroquine injection over 30 mins was not altered (control: 233 ± 47 bouts, n = 11; ablated: 240 ± 49 bouts, n = 8; two-tailed Student’s unpaired t test, t(17) = 0.1, p = 0.9).
(L and M) Schematic of textural place preference paradigm. K30 versus K150 (L); K80 versus K150 (M).
(N and O) Heatmap of mice in experimental chambers over time. K30 versus K150 (N) and K80 versus K150 (O).
(P) Preference index for rough surfaces. Ablated mice decreased preference for the rough texture chamber when textural difference was less pronounced (K80 versus K150). (K30/K150: control: 0.3 ± 0.08, n = 11; ablated: 0.1 ± 0.04, n = 10; t(19) = 2.04, p = 0.06. K80/K150: control: 0.3 ± 0.06, n = 11; ablated: 0.07 ± 0.03, n = 10; two-tailed Student’s unpaired t test; t(19) = 3, **p = 0.007).
Data are presented as mean ± SEM; p values above 0.05 not significant (n.s.).
See Figure S2.
A series of sensory tests was performed on Zic2-neuron-ablated mice and littermate controls carrying only one of the recombinases and the DTR transgene and having been subjected to the same DTx treatment. Zic2-neuron-ablated mice showed a specific sensory impairment in dynamic and static light mechanical stimulation of the plantar surface of the hindpaws. We detected reduced responses to dynamic and static brushing (Figures 2E and 2F) and in the sensitivity to gentle von Frey filament stimulations, which was seen as a mild increase in paw withdrawal threshold (Figures 2G and S2L). In contrast, Zic2-neuron-ablated mice displayed normal sensitivity to noxious mechanical stimuli, to heat and cold, and normal scratching responses evoked by chloroquine-induced itch (Figures 2H–2K). Similar results were obtained when we ablated neurons with the Zic2creERbroad line (Figures S2E–S2L).
To obtain independent evidence that Zic2 neurons mediate tactile perception, we implemented a modified texture discrimination task (Wetzel et al., 2007) that measured preference for a “rough” over a “fine” chamber floor texture. Control mice were found to spend more time on the rougher surface, whereas Zic2-neuron-ablated mice reduced their preference for the “rough” floor chamber when the textural difference was less pronounced (Figures 2L–2P). The decrease of tactile perception was remarkable considering that the whiskers were still intact in these mice. In summary, these results indicate that Zic2 spinal neurons are specifically required for innocuous light touch perception and tactile discrimination.
Zic2-Neuron-Ablated Mice Display Deficits in Fine Motor Control
Because cutaneous sensory feedback by dorsal spinal INs contributes to fine motor movements (Bourane et al., 2015; Bui et al., 2013), we addressed the role of Zic2 neurons in motor control. Zic2-neuron-ablated mice showed normal locomotor activity in an open field test, normal gross motor coordination assessed by the accelerating rotarod (Figures 3A–3C), and normal gait and limb coordination during treadmill walking (Figures S3A and S3B). However, when traversing a narrow elevated beam, Zic2-neuron-ablated mice displayed significantly more hindlimb slips than control mice (Figures 3D and 3E; Video S1). Similar motor deficits were observed when Zic2-neuron-ablated mice were challenged with crossing a narrow descending beam (Figures 3F and 3G; Video S2). Nearly identical results were obtained using the Zic2creERbroad line (Figures S3C–S3F). These results suggest that Zic2-neuron-mediated feedback to the motor system is required for corrective motor movements that presumably require intact tactile sensation in the hindpaws.
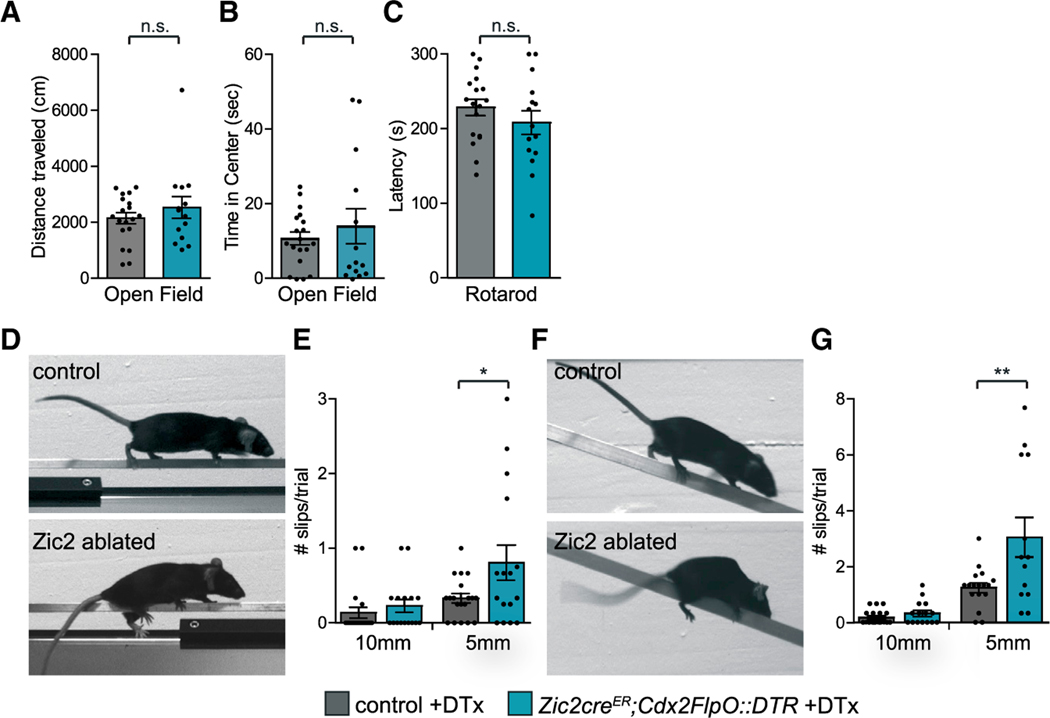
Figure 3. Fine Motor Control Is Impaired in Zic2-Neuron-Ablated Mice.
(A and B) Gross locomotor behavior in an open field arena displayed as total distance traveled (A) (control: 2,149 ± 197 cm, n = 19; ablated: 2,532 ± 388 cm, n = 14; p = 0.4) and time spent in center of arena (B) (control: 10.7 ± 1.7 s, n = 19; ablated: 14 ± 4.7 s, n = 14; p = 0.5).
(C) Gross locomotor coordination on the accelerating rotarod expressed as latency to fall (control: 229 ± 11 s, n = 18; ablated: 208 ± 16 s, n = 15; p = 0.3).
(D and F) Control and ablated mice crossing an elevated square-cross 5-mm-wide beam (D) or a descending 5-mm-wide beam (F).
(E and G) Number of hindlimb slips per single run for the elevated beams (E) (10 mm: control: 0.14 ± 0.1, n = 19; ablated: 0.23 ± 0.1, n = 16, p = 0.4. 5 mm: control: 0.33 ± 0.1, n = 19; ablated: 0.8 ± 0.2, n = 16; two-tailed Student’s unpaired t test; *p = 0.04); and for the descending beams (G) (10 mm: control: 0.18 ± 0.06, n = 19 mice; ablated: 0.33 ± 0.11, n = 15; p = 0.2. 5 mm: control: 1.25 ± 0.17, n = 17; ablated: 3.05 ± 0.71, n = 13; two-tailed Student’s unpaired t test; **p = 0.009).
Data are presented as mean ± SEM; p values above 0.05 not significant (n.s.).
Chemogenetic Activation of Zic2 Neurons Elicits Mechanical Hypersensitivity
To further evaluate Zic2 neurons, we performed gain-of-function experiments, in which we acutely activated Zic2 spinal neurons with the activating designer receptors exclusively activated by designer drugs (DREADD) receptor hM3Dq (Sciolino et al., 2016) by using intersectional genetics (Zic2creER;Cdx2FlpO;RC::hM3Dq) (Figure 4A). The activity of the hM3Dq receptor was validated by c-Fos IR, a marker for neuronal activity. We found that c-Fos IR was increased 2 h after clozapine-N-oxide (CNO) administration in hM3Dq expression as compared to control mice (Figures 4B and 4C). As previously reported for the RC::FL-hM3Dq allele, we observed faint mCherry IR in some brain regions, especially in the cerebellum and hippocampus, where Zic2cre is normally strongly expressed. This was shown to be due to a cryptic splice acceptor in the hM3Dq cDNA and to contain only mCherry mRNA and not the mRNA-encoding mCherry-hM3Dq fusion protein (Sciolino et al., 2016; data not shown).
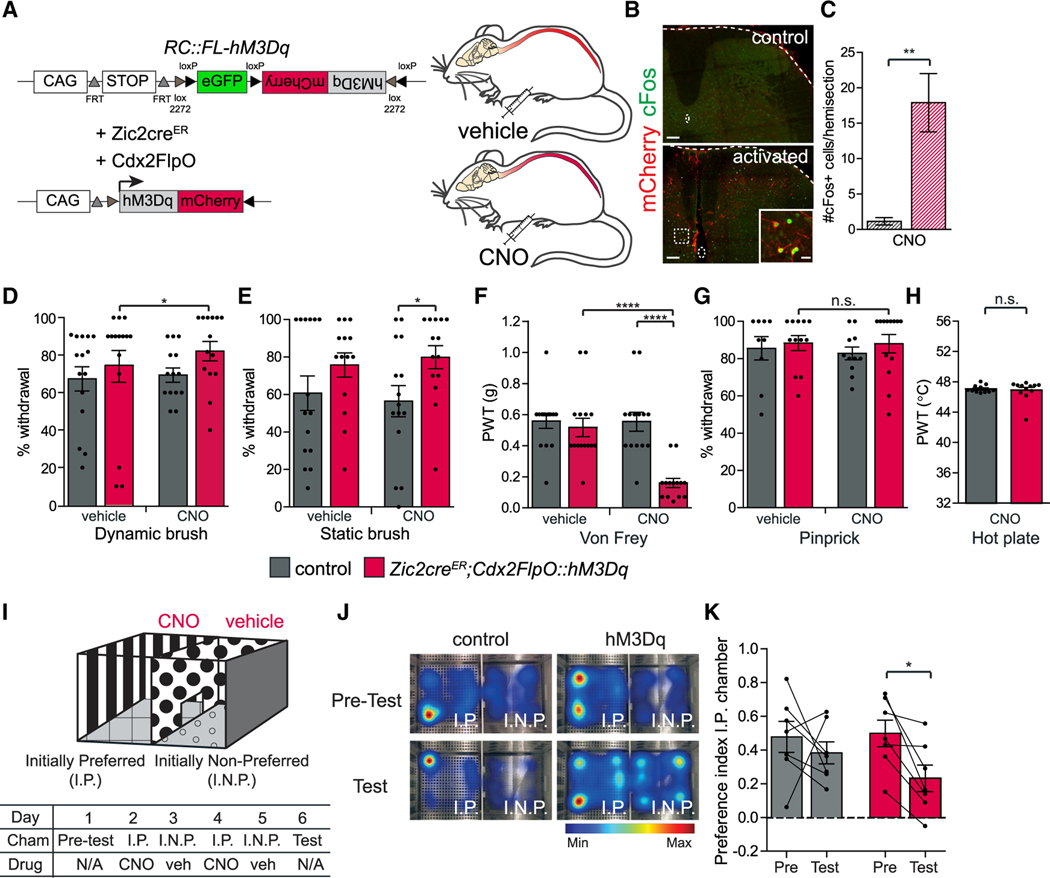
Figure 4. Activating Spinal Zic2 Neurons Increases Light Touch Sensitivity.
(A) Experimental strategy for activating Zic2cre spinal neurons. CAG promoter; FRT and loxP, recognition sites for Flp and Cre recombinases, respectively; activating DREADD hM3Dq-mCherry fusion protein; CNO, clozapine-N-oxide.
(B) Adult transverse lumbar SC sections of Cdx2FlpO::hM3Dq (control) and Zic2creERbroad;Cdx2FlpO::hM3Dq (activated) mice treated with CNO. Enlarged box: co-localization of c-Fos IR (green) with mCherry+ neurons (red). Scale bars, 100 μm; inset, 20 μm.
(C) Quantification of number of c-Fos+ cells for experiment described in (B) (control: 1.1 ± 0.5, n = 3/4 sections, 4 mice; activated: 17.9 ± 4.1, n = 3/4 sections, 5 mice; two-tailed Student’s unpaired t test, t(7) = 3.6; **p = 0.009).
(D) Activated mice show an increase in paw withdrawal to dynamic brush (control-vehicle: 68% ± 6.5%, control-CNO: 74.7% ± 8.4%, n = 15; hM3Dq-vehicle: 70% ± 3.8%, hM3Dq-CNO: 82.8% ± 5.2%, n = 14. hM3Dq-vehicle versus hM3Dq-CNO: two-tailed Student’s paired t test, t(13) = 2.6; *p = 0.02).
(E) Activated mice show an increase in paw withdrawal to static brush (control-vehicle: 60.7% ± 9.2%, control-CNO: 56.4% ± 8.3%, n = 15; hM3Dq-vehicle: 75.7% ± 6.5%, hM3Dq-CNO: 79.9% ± 6.2%, n = 14. control-CNO versus hM3Dq-CNO: two-tailed Student’s unpaired t test, t(27) = 2.2; *p = 0.03).
(F) Activated mice show a reduction in PWTs to von Frey (control-vehicle: 0.56 ± 0.05 g, control-CNO: 0.52 ± 0.06 g, n = 15 mice; hM3Dq-vehicle: 0.55 ± 0.06 g, hM3Dq-CNO: 0.16 ± 0.03 g, n = 14; hM3Dq-vehicle versus hM3Dq-CNO: two-tailed Student’s paired t test, t(13) = 6.2; ****p < 0.0001; control-CNO versus hM3Dq-CNO: two-tailed Student’s unpaired t test, t(27) = 5.3; ****p < 0.0001).
(G) Activated mice show normal withdrawal behavior in response to pinprick (control-vehicle: 85.6% ± 6.3%, control-CNO: 82.9% ± 3.5%, n = 11; hM3Dq-vehicle: 88.3% ± 4%, hM3Dq-CNO: 88% ± 4.9%, n = 13. hM3Dq-vehicle versus hM3Dq-CNO: two-tailed Student’s paired t test, t(11) = 0.4; p = 0.7).
(H) Heat sensitivity measured by paw withdrawal thresholds in dynamic hot plate (control-CNO: 47° C ± 0.1° C, n = 11; hM3Dq-CNO: 46.9° C ± 0.4° C, n = 12; two-tailed Student’s unpaired t test, t(21) = 0.4; p = 0.7).
(I) Schematic of conditioned place aversion assay. Initially preferred (I.P.) chamber and initially non-preferred (I.N.P.) chamber.
(J) Heatmap location of control and hM3Dq-expressing mice, on pre-test versus test day. hM3Dq-expressing mice show reduced preference for I.P. chamber on test versus pre-test day.
(K) Preference index toward I.P. chamber on test versus pre-test days (control pre-test: 0.48 ± 0.09, control test: 0.38 ± 0.07, n = 7; hM3D pre-test: 0.5 ± 0.08, hM3D test: 0.23 ± 0.08, n = 7. Control pre-test versus control test: two-tailed Student’s paired t test, t(6) = 0.7; p = 0.5. hM3Dq pre-test versus hM3Dq test: two-tailed Student’s paired t test, t(6) = 3.6; *p = 0.01).
Data are presented as mean ± SEM.
See Figure S4.
We performed a series of sensory tests by using a genetic control group, mice carrying only one of the recombinases plus the hM3Dq allele, and a “pharmacological” control group, mice carrying both recombinases, subjected to behavioral analysis after injection with vehicle, and subsequently injected with CNO to control for drug-induced effects (Gomez et al., 2017). Zic2-neuron-activated mice showed an increased withdrawal response to dynamic and static brush (Figures 4D and 4E) compared to the same group treated with vehicle or to the genetic control group, respectively, and a decrease in the von Frey paw withdrawal threshold compared to both genetic control group and pre-activation controls (Figures 4F, S4G, and S4H). Zic2-neuron-activated mice displayed normal sensitivity to mechanical pain and heat, based on withdrawal thresholds (Figures 4G and 4H), and an absence of signs of hypersensitivity to pain, such as guarding the paws (data not shown). Similar mechanical hypersensitivity was observed with the Zic2creERbroad line (Figures S4A–S4E and S4G). Activation of Zic2 neurons did not alter either gross or fine motor control (Figure S5), suggesting that the execution of the motor tasks was not disturbed by the excessive tactile sensation.
To assess the emotional aspects of the induced mechanical hypersensitivity, we performed a conditioned place aversion (CPA) assay. Because most mice showed an initial preference for one of the CPA chambers, we used a biased design to test whether the activation of Zic2 neurons would decrease the animals’ preference for the initially preferred (I.P.) chamber. Mice were conditioned by pairing exposure to CNO with the I.P. chamber, alternating with exposure to vehicle in the alternative chamber (Figure 4I) (Vrontou et al., 2013). On the test day, Zic2-neuron-activated mice showed a significant decrease in the time spent in the I.P. chamber that was paired with CNO (Figures 4J and 4K). Similar CPA results were obtained with the Zic2creERbroad line (Figure S4F). These data suggest that chemogenetic activation of Zic2 neurons is aversive and/or anxiogenic. In summary, these results indicate that activation of Zic2 spinal neurons leads to an increased sensitivity to light mechanical stimuli. Taken together with the loss of function results, Zic2 neurons seem to be both required and sufficient to transmit light touch sensation.
Innervation of Zic2 Neurons by Primary Sensory Neurons and Supraspinal Motor Nuclei
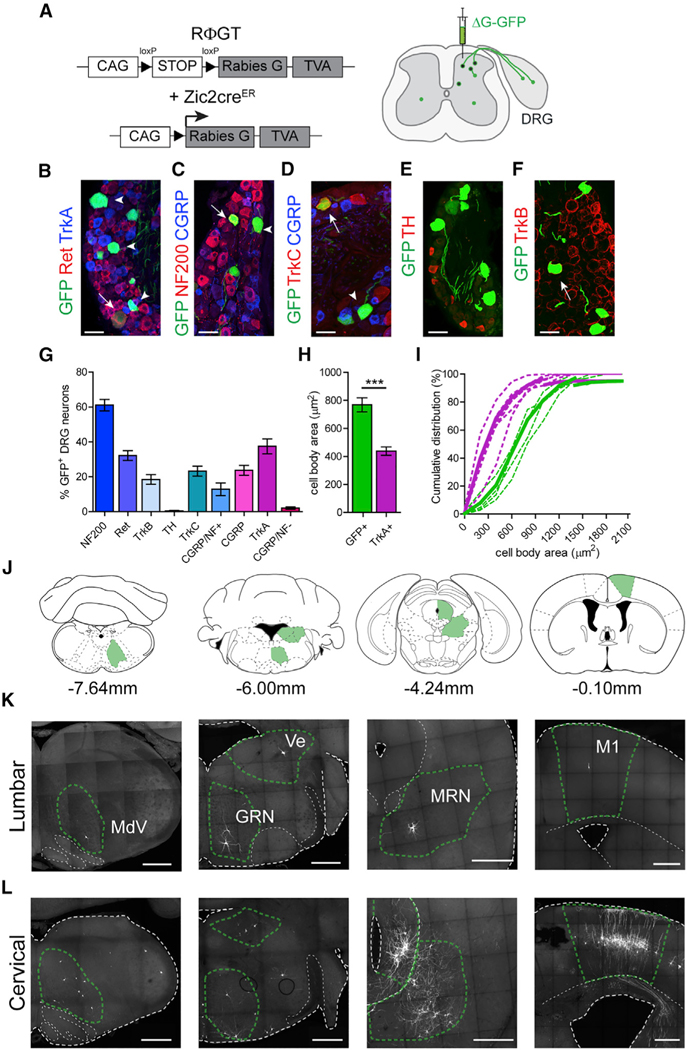
We previously suggested that Zic2 neurons receive afferent input from mechanosensory neurons (Paixão et al., 2013). In view of the function of Zic2 neurons in touch sensation, we sought to further analyze their afferent inputs. We performed retrograde monosynaptic tracing by using the EnvA pseudotyped GFP rabies virus (SADΔG-GFP) (Wickersham et al., 2007) in combination with the Cre-dependent RFGT mouse line (Takatoh et al., 2013) that expresses the auxiliary TVA and G proteins in Zic2 neurons (Figure 5A). We found numerous GFP neurons in three to four adjacent lumbar DRGs ipsilateral to the virus injection site, demonstrating monosynaptic input from primary sensory neurons onto Zic2 neurons. DRG sensory neuron types can be delineated by the expression of neurotrophic factor receptors, the diameter of the cell body, and their conduction velocity, which reflects the degree of myelination (Lallemend and Ernfors, 2012). Large-diameter myelinated LTMRs express Ret and TrkB; large proprioceptive neurons express TrkC; and small unmyelinated neurons involved in nociception, thermoception, pruriception, and gentle touch (C-fibers) express TrkA. The molecular characterization of the GFP+ DRG neurons revealed that 61.1% ± 3.3% of these neurons were myelinated (NF200+), including 12.8% ± 3.6% peptidergic neurons (CGRP+/NF200+). Only 2.1% ± 0.7% were unmyelinated peptidergic (CGRP+/NF200−) nociceptive neurons. A total of 32.2% ± 2.8% were c-Ret+, a marker of Aβ-LTMRs; 18.7% ± 3.9% were TrkB+, a marker of Aδ-LTMRs; 37.5% ± 4.2% were TrkA+, traditionally associated with nociceptive neurons; 23.7% ± 2.8% were CGRP+; and 23.2% ± 2.8% were TrkC+, which labels both proprioceptive and LTMRs. We failed to detect colocalization with tyrosine hydroxylase (TH), C-low-threshold mechanoreceptor (C-LTMR) neurons (0.35% ± 0.3%) (Figures 5B–5G). The mean soma size of GFP+ DRG neurons was consistent with Aβ-LTMR neurons that have large cell body sizes and was significantly different from small-diameter TrkA neurons (Figures 5H and 5I) (Luo et al., 2009; Molliver et al., 1995).
Figure 5. Innervation of Spinal Zic2 Neurons by Primary Sensory and Supraspinal Neurons.
(A) Experimental strategy for monosynaptic retrograde tracing of Zic2cre spinal neurons. CAG promoter; loxP, recognition sites for Cre recombinase. An EnvA-pseudotyped G-deleted eGFP virus (ΔG-GFP) was injected intraspinally at L2/L3 or C2 levels in P7 pups (right panel).
(B–F) Sections of P15 lumbar DRGs showing monosynaptic Zic2 input neurons, and immuno-logical analysis of the sensory neuron subtype markers indicated. Arrows indicate double-labeled neurons with marker shown in red; arrowheads indicate double-labeled neurons with marker shown in blue. Ret and TrkA (B), NF200 and CGRP (C), TrkC and CGRP (D), TH (E), and TrkB (F).
(G) Sensory neuron marker abundance among monosynaptic Zic2 input neurons (n = 3/6 sections, 3–7 mice).
(H) Cell body area of monosynaptic DRG neurons (GFP+) compared to TrkA+ neurons (GFP: 769 ± 50 μm2, n = 42 to 115 neurons, 5 mice; TrkA: 439 ± 30 μm2, n = 120 to 150 neurons in 3 sections from 5 mice each; two-tailed Student’s unpaired t test, t(8) = 5.7; ***p = 0.0005).
(I) Cumulative distribution of cell body area of monosynaptic Zic2 input DRG neurons (green) compared to TrkA+ neurons (magenta). Dotted lines indicate results of single animals. Solid lines indicate average distribution.
(J) Coronal-section planes with distance from Bregma of images shown in (K) and (L).
(K and L) P15 brain sections showing monosynaptic Zic2 input neurons. MdV, medullary reticular formation ventral part; Ve, Vestibular nucleus; GRN, gigantocellular reticular nucleus; MRN, midbrain reticular nucleus; M1, primary motor cortex. Distribution of supra-spinal monosynaptic inputs of lumbar (K) and cervical (L) Zic2cre spinal neurons.
Scale bars, 50 μm (B–F) and 500 μm (K and L). Data are presented as mean ± SEM.
We also found that Zic2 neurons received monosynaptic inputs from a wide range of brain regions, most of which are known to take part in descending motor pathways (Figures 5J–5L). Prominent examples were the brainstem nucleus medullary reticular formation ventral part (MdV), shown to be important for skilled motor tasks (Esposito et al., 2014); the gigantocellular reticular nucleus (GRN), which contributes to the reticulospinal tract and is important for motor modulation (Bouvier et al., 2015; Brownstone and Chopek, 2018; Capelli et al., 2017; Liang et al., 2016); the vestibular nuclei of the brainstem (Ve), which play a key role in the maintenance of balance and postural stability (Basaldella et al., 2015; Murray et al., 2018); the midbrain reticular nucleus (MRN), containing both the mesencephalic locomotor region (MLR) that plays a prominent role in the initiation and modulating the speed of locomotion (Caggiano et al., 2018; Capelli et al., 2017; Liang et al., 2012b), and the red nucleus that is important for skilled limb movements (Liang et al., 2012a); and the primary motor cortex (M1), where we observed labeled pyramidal neurons in layer V corresponding to corticospinal projection neurons (CSTs) that have been shown to be involved in goal-directed movements (Moreno-López et al., 2016; Ueno et al., 2018). We observed a greater representation in the number of neurons that target the cervical Zic2 population (Figure 5L) compared to the lumbar population (Figure 5K), which might reflect an anatomical bias of descending motor pathways toward the cervical spinal cord for the fine motor control of forelimb movements.
Zic2 INs Form Synaptic Contacts with Motor Neurons
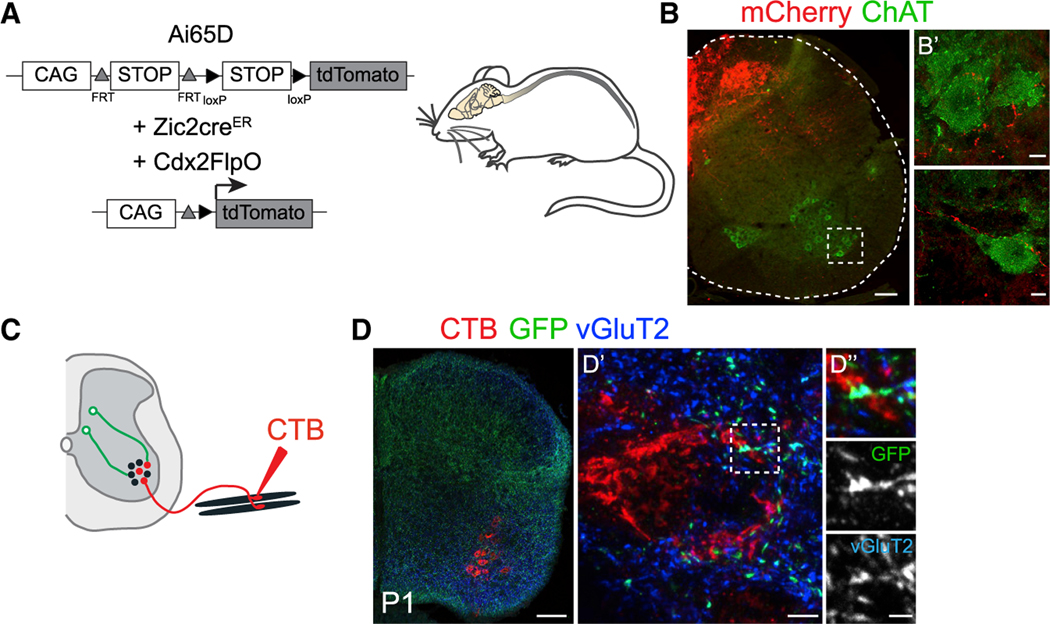
To further investigate the circuitry by which Zic2 neurons modulate tactile perception and fine motor control, we anatomically mapped the axonal projections of these neurons. For this, we used the intersectional Ai65D Flp- and Cre-dependent tdTomato reporter (Madisen et al., 2015) (Figure 6A) to restrict expression of tdTomato to Zic2 spinal neurons. We observed a proportion of Zic2 axons extending toward the ventral spinal cord and surrounding ChAT+ motor neurons (Figure 6B). Using the Cre-dependent synaptophysin-GFP (Taulox-STOP-lox-SynGFP) reporter (Tripodi et al., 2011), we observed that Zic2 neurons formed excitatory pre-synaptic boutons (GFP/vGluT2+) directly onto motor neurons, which were retrogradely labeled by injection of the CTB tracer into hindlimb muscles (Figures 6C and 6D). These tracing studies provide evidence that a subpopulation of Zic2 neurons likely relays information from cutaneous LTMRs to the motor system.
Figure 6. Zic2 Spinal Neurons Synapse with Spinal Motor Neurons.
(A) Intersectional strategy to exclusively visualize Zic2creER spinal neurons. CAG promoter; FRT and loxP, recognition sites for Flp and Cre recombinases, respectively.
(B) Transverse section of a P15 lumbar SC Zic2-creER;Cdx2FlpO::Ai65D immunolabeled with mCherry (red) and ChAT antibodies (green). Note tdTomato+ axons surrounding motor neurons (B’).
(C) Experimental strategy to visualize Zic2creER pre-synaptic terminals (green) and retrogradely labeled spinal motor neurons with CTB injections in the quadriceps muscle (red).
(D) Transverse section of Zic2creER; Taulox-STOP-lox-SynGFP lumbar SC retrogradely traced with CTB (red) and immunolabeled with GFP (green) and vGluT2 antibodies (blue). SynGFP marks Zic2+ terminals co-localizing with vGluT2 on CTB labeled motor neurons (D’ and D”).
Scale bars, 100 mm (B and D), 10 mm (B’), 5 mm (D’), and 2 mm (D”).
Zic2 Neurons Contribute to the PSDC Pathway
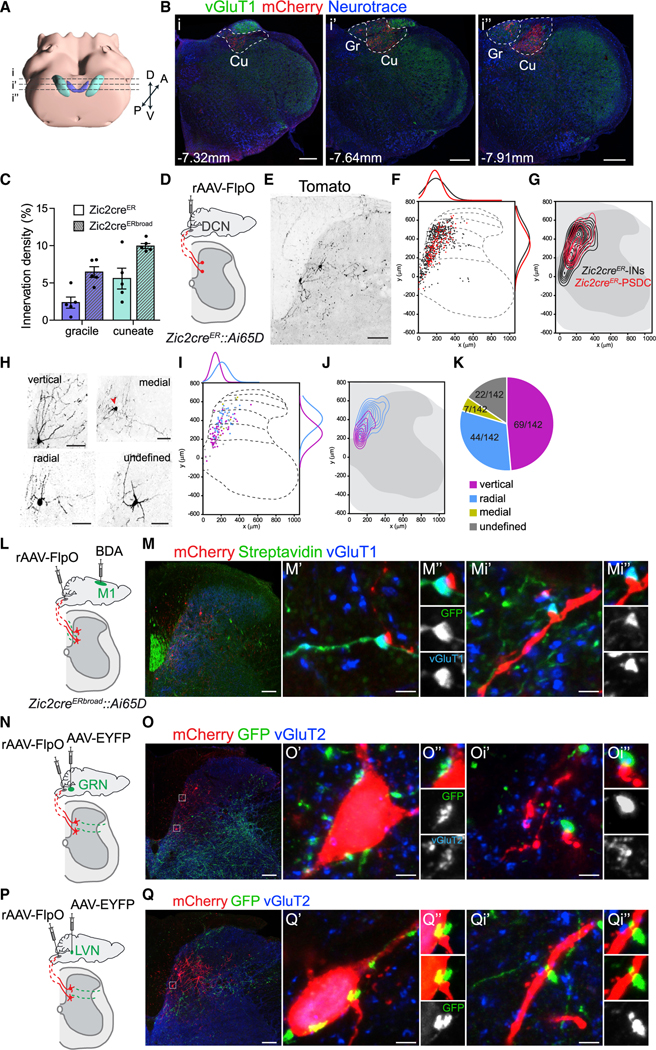
When we visualized the long-range Zic2 axon projections to the brain, we observed dense efferent fields exclusively in the DCN. To assess the contribution of Zic2 neurons to the PSDC pathway, we examined the innervation density of the two DCN nuclei, gracile and cuneate, in both Zic2cre lines. We found that Zic2 PSDC neurons predominantly innervated the cuneate nucleus, which receives the terminals from the cervical spinal segments, and that Zic2 axons covered between 2%–10% of the areas of the two nuclei depending on which Zic2cre line was used for the analysis (Figures 7A–7C and S6A–S6E). In comparison, the axons of Lbx1cre neurons, which represent the majority of neurons in the dorsal horn LTMR-RZ (Abraira et al., 2017), reached 40% coverage (data not shown), suggesting that Zic2 neurons comprise a substantial fraction of all PSDC neurons.
Figure 7. Characterization of Zic2-PSDC Neurons.
(A) 3D view of the hindbrain, gracile (Gr, purple), and cuneate (Cu, teal) nuclei, adapted from the Allen Brain Explorer (beta) (Lau et al., 2008). Stippled lines represent section planes of anterior (i), medial (i’), and posterior (i”) regions of the DCN shown in (B).
(B) Coronal medulla sections of adult Zic2creER;Cdx2FlpO::Ai65D showing mCherry (red) and vGluT1 (green) IR counterstained with Neurotrace (blue) in the anterior (i), medial (i’), and posterior (i”) medulla. Distances from Bregma indicated. Gr and Cu nuclei highlighted by stippled lines.
(C) Innervation density of Zic2creER and Zic2creERbroad PSDC axons in Gr and Cu nuclei, represented as number of mCherry+ pixels/total amount of pixels per nucleus averaged from the different regions analyzed in (B) (Zic2creER gracile: 2.3% ± 0.7%; cuneate: 5.6% ± 1.4%; Zic2creERbroad gracile: 6.4% ± 0.7%; cuneate: 9.9% ± 0.4%, n = 5 mice, 1/2 sections per region).
(D) Experimental strategy to specifically visualize the Zic2cre spinal neuron subpopulation projecting to the DCN.
(E) Transverse SC section after transduction with retroAAV-FlpO (rAAV-FlpO) in the DCN of Zic2creER;Ai65D. Retrogradely labeled neurons visualized by tomato IR and shown in black.
(F) Spatial distributions of individual spinal Zic2creER-tdTomato neurons (black) and Zic2creER-tdTomato PSDC neurons (red) (0,0 indicates the central canal). Comparison of spatial frequency distributions in dorsal-ventral (right) and medial-lateral axis (top), represented as non-linear regressions.
(G) Contour density plots of all Zic2creER-tdTomato neurons (Zic2creER-INs, black) and Zic2creER-tdTomato PSDC neurons (red).
(H) Example images of Zic2 PSDC neurons showing the different morphological categories observed.
(I) Spatial distributions of different Zic2creER-tdTomato PSDC neuron categories. Comparison of spatial frequency distributions in the dorsal-ventral axis (right) and medial-lateral axis (top) of the two main neuronal categories: vertical (magenta) and radial (cyan) represented as non-linear regressions.
(J) Contour density plots of vertical Zic2creER-tdTomato+ PSDC neurons (magenta) and radial Zic2creER-tdTomato PSDC neurons (cyan).
(K) Morphological profile of Zic2 PSDC neurons.
(L, N, and P) Experimental strategy to specifically visualize synaptic connections from different brain centers, namely, motor cortex (M1) (L), GRN (N), and lateral vestibular nucleus (LVN) (P), onto Zic2-PSDC neurons marked by the intersectional Ai65D-tdTomato reporter and retrograde transduction with rAAV-FlpO in DCN. BDA, biotinylated dextran amine.
(M) Transverse section of a cervical SC after BDA injection in M1. Zic2 PSDC neurons labeled by mCherry (red), and corticospinal tract axons and pre-synaptic boutons labeled with streptavidin (green). Note GFP/vGluT1+ (blue) boutons on dendrites of Zic2 PSDC neurons (M’–Mi”).
(O) Transverse section of a cervical SC after transduction with AAV-EYFP in GRN. PSDC neurons labeled by mCherry (red), and reticulospinal axons and pre-synaptic boutons labeled with GFP (green) IR. Note GFP/vGluT2+ (blue) boutons on the soma (O’ and O”) and dendrites (Oi’ and Oi”) of Zic2 PSDC neurons.
(Q) Transverse section of a cervical SC after transduction with AAV-EYFP in LVN. PSDC neurons abelled by mCherry (red), and vestibulospinal axons and pre-synaptic boutons abelled with GFP (green) IR. Note GFP+ boutons on the soma (Q’ and Q”) and dendrites (Qi’ and Qi”) of Zic2 PSDC neurons. Scale bars, 300 μm (B), 100 μm (E, M, O, and Q), and 3 μm (M’, Mi’, O’, Oi’, Q’, and Qi’).
Data are presented as mean ± SEM.
See Figure S6.
To anatomically characterize Zic2 PSDC neurons, we injected a retrograde AAV-FlpO virus (rAAV-FlpO) (Tervo et al., 2016) into the DCN in mice carrying the Zic2cre and the intersectional Ai65D Flp- and Cre-dependent tdTomato reporter (Figure 7D). Using both Zic2cre lines, we observed that Zic2 PSDC neurons were located in a medial column in laminae III–V. A comparison of the laminar distribution of Zic2 PSDC neurons to the entire Zic2 neuron population revealed a remarkable spatial overlap of Zic2creER PSDC, Zic2creERbroad PSDC, and Zic2creER spinal neurons (Figures 7E–7G, S6F, and S6G). Conversely, the fraction of Zic2creERbroad spinal neurons that extends to more lateral parts of laminae III–IV did not contribute to the PSDC population (Figures S6F and S6G).
We characterized the Zic2 PSDC neurons morphologically, according to published criteria (Grudt and Perl, 2002; Yasaka et al., 2010) and cell body position. We found that Zic2 PSDC neurons fall into two main categories, namely, vertical (48.6%) and radial (31%) (Figures 7H–7K), which interestingly segregated in the dorso-ventral axis. We observed that most of the radial Zic2 PSDC neurons were located in laminae III–IV, whereas the most abundant vertical categories were located in the deeper laminae IV–V (Figures 7I and 7J). Identical results were observed using the Zic2-creERbroad line (Figures S6H–S6J). This indicates an anatomical heterogeneity among Zic2 PSDC neurons, which may reflect functional heterogeneity.
We occasionally observed processes extending to the ventral spinal cord, but failed to identify axonal branches synapsing on motor neurons (data not shown), implying that the subpopulation of Zic2 neurons relaying sensory information to the motor system is distinct from the Zic2 PSDC population.
To begin investigating the supraspinal input connectivity of Zic2 PSDC neurons, we combined anterograde tracings by using biotinylated dextran amine (BDA) or AAV-enhanced yellow fluorescent protein (EYFP) injections from different identified supraspinal nuclei with the previously described strategy to specifically label Zic2 PSDC neurons (Figures 7L, 7N, and 7P). We observed that Zic2 PSDC dendritic processes received synaptic input from CST axons (Figure 7M), as described for PSDC neurons (Abraira et al., 2017). Interestingly, reticulospinal tract axons, originating from the GRN, formed excitatory pre-synaptic boutons onto Zic2 PSDC cell bodies and dendritic processes (Figure 7O). Similarly, we also observed vestibulospinal tract pre-synaptic boutons, from the lateral vestibular nucleus (LVN), onto Zic2 PSDC cell bodies and dendritic processes (Figure 7Q). These results suggest that Zic2 PSDC neurons might be involved in the sensorimotor integration to couple voluntary actions with posture and locomotion.
Activation of Zic2 PSDC Neurons Induces Mechanical Hypersensitivity
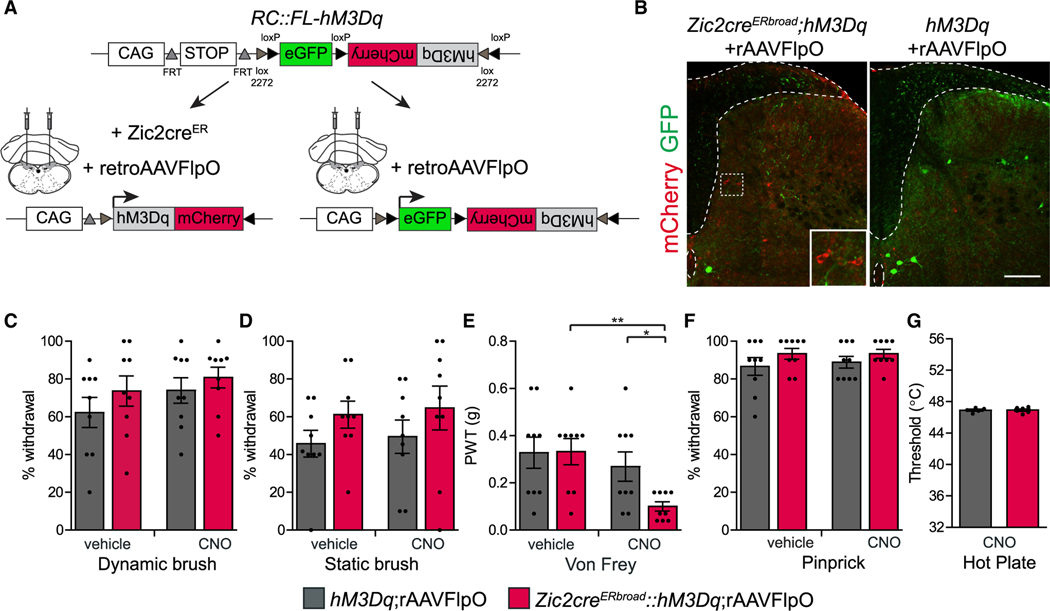
To evaluate the functionality of the Zic2 PSDC projection in the fine touch circuit, we injected the rAAV-FlpO virus in the DCN of mice carrying the Zic2cre and the activating hM3Dq trans-genes or hM3Dq alone as the control. Because the characterization of Zic2 PSDC neurons did not seem to differ between the two Zic2cre lines, we used the Zic2creERbroad line to increase the probability of targeting Zic2 PSDC neurons (Figures 8A and 8B). This retrograde viral strategy led to hM3Dq expression in approximately one-third of Zic2+ neurons (Figure S7A). Upon activation of Zic2 PSDC neurons with CNO, mice did not show higher sensitivity to dynamic or static brush (Figures 8C and 8D). However, they showed an increase in sensitivity to stimulations with gentle von Frey filaments, compared to both genetic control group and pre-activation controls (Figures 8E and S7B), recapitulating the results observed with the global activation of spinal Zic2 neurons. As expected, Zic2 PSDC-activated mice displayed no changes to mechanical pain or sensitivity to heat (Figures 8F and 8G). These results confirm that Zic2 neurons comprise a significant population of PSDC spinal neurons that receive and transmit light touch information to higher brain centers by the DCN.
Figure 8. Activating Zic2 PSDC Neurons Increases Light Touch Sensitivity.
(A) Experimental strategy to specifically activate Zic2-PSDC neurons. CAG promoter; FRT and loxP, recognition sites for Flp and Cre recombinases, respectively; eGFP is expressed upon Flp recombination; activating DREADD hM3Dq-mCherry fusion protein is expressed upon Flp and Cre recombination.
(B) Transverse sections of lumbar SCs after transduction with rAAV-FlpO in the DCN of the medulla of Zic2-CreERbroad;hM3Dq (left) and control hM3Dq (right) mice immunolabeled with mCherry (to visualize hM3Dq, see also higher magnification inset) and GFP antibodies (to visualize unrecombined loxP-flanked eGFP fragment). Dorsal funiculus and central canal indicated with stippled lines. Scale bar, 200 μm.
(C) Response of Zic2 PDSC-activated mice to dynamic brush, represented as percentage of paw withdrawal (control-vehicle: 62.2% ± 7.9%, control-CNO: 73.9% ± 6.7%, n = 9; hM3Dq-vehicle: 73.6% ± 8.0%, hM3Dq-CNO: 80.8% ± 5.5%, n = 9. hM3Dq-vehicle versus hM3Dq-CNO: two-tailed Student’s paired t test, t(8) = 1.1; p = 0.3).
(D) Response of Zic2 PDSC-activated mice to static brush, represented as percentage of paw withdrawal (control-vehicle: 45.7% ± 7.1%, control-CNO: 49.5% ± 8.8%, n = 9; hM3Dq-vehicle: 61.1% ± 7.2%, hM3Dq-CNO: 64.7% ± 11.6%, n = 9. hM3Dq-vehicle versus hM3Dq-CNO: two-tailed Student’s paired t test, t(8) = 0.6; p = 0.6).
(E) Zic2 PSDC-activated mice show a reduction in PWTs to von Frey (control-vehicle: 0.33 ± 0.07 g, control-CNO: 0.27 ± 0.06 g, n = 9 mice; hM3Dq-vehicle: 0.33 ± 0.06 g, hM3Dq-CNO: 0.1 ± 0.02 g, n = 9; hM3Dq-vehicle versus hM3Dq-CNO: two-tailed Student’s paired t test, t(8) = 3.7; **p = 0.006. control-CNO versus hM3Dq-CNO: two-tailed Student’s unpaired t test, t(16) = 2.6; *p = 0.02).
(F) Zic2 PSDC-activated mice show normal withdrawal behavior in response to pinprick (control-vehicle: 86.7% ± 4.7%, control-CNO: 88.9 ± 3.1%, n = 9; hM3Dq-vehicle: 93.3% ± 2.9%, hM3Dq-CNO: 93.3% ± 2.4%, n = 9. hM3Dq-vehicle versus hM3Dq-CNO: two-tailed Student’s paired t test, t(8) = 0; p = 1).
(G) Zic2 PSDC-activated mice show normal heat sensitivity measured by PWTs in the dynamic hot plate (control-CNO: 46.9° C ± 0.1° C, n = 5; hM3D-CNO: 46.9° C ± 0.1° C, n = 6; two-tailed Student’s unpaired t test, t(9) = 0.04; p = 0.97).
Data are presented as mean ± SEM.
See Figure S7.
DISCUSSION
This study identifies a novel class of excitatory neurons in the dorsal spinal cord that plays an important role in mediating innocuous touch. We show that Zic2 neurons are both required and sufficient to transmit light touch sensation. A subpopulation of Zic2 neurons constitute ascending output projections to the brainstem DCNs, establishing these neurons as PSDCs. We find that a proportion of Zic2 axons form synaptic boutons onto spinal motor neurons and Zic2 neurons are required for corrective motor movements. Taken together, Zic2 neurons likely integrate sensory input from cutaneous LTMRs with descending signals from the cortex and brainstem motor nuclei to transmit contextualized touch information back to brainstem sensory nuclei and to generate the motor output needed for skilled limb movements.
Processing of Innocuous Touch by Zic2 INs
Ablation of Zic2 spinal neurons impaired the sensation of dynamic and static touch and reduced the ability of the mice to discriminate between different textures. In contrast, Zic2-neuron-ablated mice showed normal responses to noxious mechanical, thermal, and chemical itch stimuli, indicating a role for Zic2 neurons in processing sensory inputs in a modality-specific manner. These results, and previous work (Bourane et al., 2015; Ma, 2010), support the existence of “labeled lines” of INs dedicated to touch in the spinal cord.
We found that Zic2 neurons partially overlap with RORα neurons within lamina III, a population of excitatory INs required for dynamic and static touch. Similar to Zic2 neurons, RORα INs are involved in corrective motor behavior. Unlike Zic2 neurons, RORα IN-ablated mice showed normal sensitivity to von Frey filaments, suggesting that this type of touch perception may require Zic2 neurons in deep layers IV–V. Certainly, the PSDC fraction of Zic2 neurons is distinct from RORα INs, which appear to be exclusively local. Instead, some of the RORα INs project onto PSDC neurons (Bourane et al., 2015) and might act upstream of Zic2 neurons to mediate tactile perception.
CCK excitatory INs were shown to mediate tactile discrimination without affecting responses to noxious heat (Abraira et al., 2017). Although a significant proportion of CCK INs localize within laminae II–III, where Zic2 and RORα neurons reside, the CCK IN population extends to many other regions of the dorsal horn, including superficial laminae that receive nociceptive input. Hence, the contributions that CCK INs make to the transmission of other sensory modalities remain to be determined. Other excitatory INs (Sst and vGluT3) likely represent multiple functionally distinct populations that play roles in touch and pain sensitivities (Cheng et al., 2017; Duan et al., 2014; Peirs et al., 2015). Excitatory INs are under constant control by inhibitory INs to prevent cross-modal activation and aberrant spread of excitation. Several populations of inhibitory INs were previously shown to modulate touch perception (Cui et al., 2016; Koch et al., 2017; Petitjean et al., 2015), either acting pre-synaptically onto primary afferents or post-synaptically onto local excitatory INs. Which of these inhibitory INs modulate the activity of Zic2 neurons remains to be determined.
Zic2 PSDC Neurons Contribute to Processing of Innocuous Touch
We and others have previously characterized Zic2 neurons in the embryonic spinal cord by virtue of their ipsilateral ascending axon projections within the dorsal column (Escalante et al., 2013; Paixão et al., 2013). Here, we show that Zic2cre-expressing neurons maintain this projection in the adult and that chemogenetic activation of this Zic2 subpopulation leads to mechanical hypersensitivity. Zic2, thus, represents the first genetic marker for an excitatory neuron subpopulation that develops into functional PSDC neurons. We believe it is a minor subpopulation because the total fraction of supraspinal projecting neurons originating in the LTMR-RZ is thought to be less than 2% of neurons in this region (Abraira et al., 2017).
Chemogenetic activation of Zic2 PSDC neurons led to hypersensitivity toward gentle von Frey filaments but left the responses to brush stimuli unaltered, unlike activation of the global Zic2 spinal population. This could be due to the smaller numbers of neurons targeted by the viral approach used or may reflect a real biological distinction between the two populations. There are subtle differences between static brush and single von Frey filaments, such as receptive field size, duration of stimulation, and force applied. Interestingly, we have also described that Zic2 PSDC neurons have distinct morphologies that segregate in different laminae of the dorsal horn, similar to pioneering work on cat PSDC neurons (Brown and Fyffe, 1981; Enevoldson and Gordon, 1989). Further work is required to address whether this morphological heterogeneity reflects a functional distinction.
Zic2 Neurons Are Required for Corrective Motor Movements
Our functional analysis of Zic2 neurons adds to the growing evidence that cutaneous sensory feedback is essential for the corrective movements required for balance and proper foot placement on an irregular surface. These sensory feedback signals are integrated in the deep dorsal horn with descending motor signals to modify the locomotor CPG. To date, studies of sensory integration in spinal motor circuits have largely focused on the influence of proprioceptive inputs on spinal neurons, but cutaneous afferents also regulate the output of spinal motor circuits most notably on the control of locomotion and corrective movements (Panek et al., 2014; Rossignol et al., 2006). Elimination of cutaneous input from the hindpaws in cats did not compromise their ability to walk over ground but impaired their ability to walk on a ladder or an inclined treadmill (Bouyer and Rossignol, 2003). Similarly, human studies suggest that cutaneous reflexes from tactile input to the plantar aspect of the foot are important for maintaining stability during challenging walking conditions (Panek et al., 2014; Zehr and Stein, 1999). Recently, classes of excitatory dorsal INs have been shown to receive LTMR input and to directly synapse and modulate the output of motor neurons. Inactivation of such INs leads to deficits in motor tasks, including corrective movements during locomotion (Bourane et al., 2015; Bui et al., 2013). In agreement with those observations, Zic2-neuron-abla-ted mice display defects in corrective movements in the elevated beam paradigm, a challenging motor task that likely requires a high degree of cutaneous sensory feedback. We also show that Zic2 neurons form excitatory pre-synaptic boutons onto spinal motor neurons, but it remains to be shown whether Zic2 neurons exert their influence on the motor output by directly influencing the action of motor neurons or by another class of spinal INs.
From an anatomical perspective, it is interesting to note that many Zic2 neurons lie at a medial position in the deep dorsal horn, an important area for motor control. Many premotor neurons (Levine et al., 2014; Tripodi et al., 2011), including “reflex-encoder” sensory relay neurons (Levinsson et al., 2002; Schouenborg et al., 1995), and so-called motor synergy encoder neurons localize to this area of the spinal cord. Populations of INs in this region represent a central node for voluntary and reflexive movements. Zic2 neurons only partially match the molecularly identified relay neurons, which are predominantly inhibitory, including Tcfap2β and SatB2 INs (Hilde et al., 2016; Levine et al., 2014). It remains to be seen if inhibitory relay neurons and Zic2 neurons are part of the same circuit or belong to distinct circuits that control different types of motor behavior.
Monosynaptic Inputs to Zic2 Neurons
Zic2 neurons receive direct synaptic input from multiple LTMR subtypes, suggesting that they process multiple types of mechanosensory input from the skin. This is in agreement with previous observations on LTMR-RZ neuron subtypes that were shown to receive inputs from at least one to three LTMR subtypes (Abraira et al., 2017; Bourane et al., 2015).
We also observed direct inputs to Zic2 spinal neurons from different supraspinal regions, and notable examples were brainstem nuclei, such as the medullary reticular formation, GRN, vestibular nucleus, the red nucleus, and the motor cortex. Common movements, such as reaching and grasping an object or stepping involve complex neuronal calculations to select the appropriate muscles and precisely control the timing of their contractions to achieve the desired outcome. This motor coordination involves many regions in the CNS, including the motor cortex, red nucleus, basal ganglia, brainstem, cerebellum, peripheral sensory system, and spinal neurons (Ferreira-Pinto et al., 2018). These neuronal pathways ultimately converge onto motor neuron pools, often by spinal cord INs. Many of the nuclei that monosynaptically connect to Zic2 neurons are involved in different aspects of motor control (Basaldella et al., 2015; Caggiano et al., 2018; Murray et al., 2018; Ueno et al., 2018). Several of these descending motor control pathways also interact directly with the ascending PSDC pathway. This presents an advance in the understanding of how sensorimotor integration is achieved in the dorsal spinal cord and, in particular, at the level of PSDC neurons. The somatosensory cortex had been proposed as a source of descending influence on PSDC neurons (Abraira et al., 2017), and excitatory responses of PSDC neurons to cutaneous input were shown to be modulated by noradrenaline and serotonin in the cat (Fleetwood-Walker et al., 1985; Jankowska et al., 1997), suggesting that brainstem nuclei might influence this pathway. In the future, it would be important to understand in which contexts the reticulospinal, vestibulospinal, and corticospinal pathways interact with the cutaneous sensory pathway in the spinal cord. We speculate that it could act on sensory afferent gating or on mechanisms for anticipating cutaneous sensory inputs generated by voluntary movements. For instance, motor cortical modulation of cutaneous reflexes has been proposed to act by a spatial convergence of corticospinal and cutaneous afferent activity mediated by distinct subpopulations of spinal INs (Bretzner and Drew, 2005). Corticospinal neurons, which have long been recognized to control goal-directed movements, were recently shown to modulate tactile sensory processing in the spinal cord in a feedback loop that facilitates light-touch-evoked activity of CCK INs in the deep dorsal horn (Liu et al., 2018).
Conclusions
Our ability to feel tactile sensations is crucial for many aspects of our life, and still, surprisingly little is known about the underlying neuronal mechanisms of touch perception. The spinal circuits described in this study represent a small but important fraction of the circuits involved in the complex and multifaceted sensation of touch. As circuit dissection proceeds, the connectivity and function of different players in the dorsal horn will be uncovered as well as the interaction of different somatosensory modalities and the integration of sensory feedback with motor control.
STAR★METHODs
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Rüdiger Klein (rklein@neuro.mpg.de).
The Zic2creER mouse line generated in this study has been submitted to MMRRC, and is available upon request to the Lead Contact, until deposition and distribution are available.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse lines
For the generation of Zic2creER mice, a BAC homologous recombination method was used. The BAC clone RP23–158G6 (CHORI) was targeted with a CreERT2 expression cassette containing the polyadenylation site (polyA) from the SV40 early region (Feil et al., 1997), followed by a kanamycin resistance cassette. The BAC homologous recombination cassette was assembled in the pBK-CMV vector (Stratagene) as follows. Homology arms A and B flanking mouse Zic2 exon 1 were synthesized by PCR using the following primer sets: 5’-TATGTCGACCCTGGTCCGCCGGCAGTACAA-3′, and 5′-ACGGTCAGTAAATTGGACATGGCCAGC GCGCCCGGCCG-3′ (arm A); 5′-TACAGTACTGCGCACATGGGCGCCTTCAAGC-3′, 5′-AAGTGACCACCCGGGTGGATGTTCATAT TCATAGGGC-3′ (arm B). First, pBK-CMV was digested with ScaI/BstXI to insert homologous arm B. Second, the kanamycin resistance cassette (Gene Bridges GmbH) was cloned into the SpeI/ScaI site before the arm B in pBK-CMV. Then, a single fragment containing the homologous arm A and CreERT2polyA cassette was obtained by overlap extension PCR and inserted into the SalI/SpeI site in pBK-CMV before the kanamycin resistance cassette. This targeting vector was digested with SalI/BstXI followed by purification of the insert on a 0.8% agarose gel. Homologous recombinant BACs were obtained using established methods (Chaveroche et al., 2000), screened by PCR and verified by Southern blotting. Modified BAC DNA was prepared using the large construct DNA purification kit, NucleoBond ® Xtra BAC (Macherey-Nagel), linearized with AscI and purified through a Sepharose separation column (Johansson et al., 2010). The BAC DNA (4.9 ng/μl) was injected into pronuclei of fertilized oocytes of C57BL/6 mice. BAC transgenic mice were identified by PCR. The previously described mouse lines were used in this study: Taulox-stop-loxSynGFP (Tripodi et al., 2011); RC::FL-hM3Dq (Sciolino et al., 2016); Tauds-DTR (Britz et al., 2015); Cdx2-FlpO (Britz et al., 2015); Ai9 td-Tomato (B6.Cg-Gt(ROSA) 26Sortm9(CAG-tdTomato)Hze/J) (Madisen et al., 2010); Ai14 td-tomato (Madisen et al., 2010) were obtained by crossing Ai9 td-tomato with the R26FC31 (B6;129S4-Gt(ROSA)26Sortm3(phiC31*)Sor/J) mouse line to remove the AttB/AttP-flanked sequence (Raymond and Soriano, 2007) Ai65D RC::FL-tdTomato (B6;129S-Gt(ROSA)26Sortm65.1(CAG-tdTomato)Hze/J) (Madisen et al., 2015); RFGT (B6;129P2-Gt(ROSA)26Sortm1(CAG-RABVgp4,-TVA)Arenk/J) (Takatoh et al., 2013). All lines were backcrossed to C57BL/6 mice at least twice.
Animal statement
All procedures were approved by the government of Upper Bavaria (License number 55.2–1-54–2532-130–2015) and animals were kept and used in accordance with regulations from the government of Upper Bavaria.
For behavioral experiments, mice were housed on an inverted 12 hr light/dark cycle with ad libitum access to food and water. Animals were acclimated to the inverted light cycle for a week before the beginning of testing. Behavioral tests were conducted during the dark phase. All control animals were littermates, and thus all had the same genetic background and subjected to the same treatments.
Induction of Cre was done on E14.5 pregnant females. Histological experiments were done on E16.5- E18.5, P1, P7, P15 and adult animals. Monosynaptic rabies was injected in P7 animals. Motorneuron tracings were done in P1 animals. Stereotaxic surgeries were done in 6–8 weeks old animals. Adult mice (P56–90) were used for behavior experiments. Both male and female mice were used.
METHOD DETAILS
Virus
The EnvA G-deleted rabies-GFP (Wickersham et al., 2007) and AAVretro-Ef1a-FlpO (Tervo et al., 2016) were produced by the Viral Vector Core of the Salk Institute. AAV5-hSyn-EYFP was produced at the Gene Therapy Center Vector Core at the University of North Carolina Chapel Hill.
Histology
Post-natal animals were deeply anesthetized with ketamine/xylazine (100 mg/kg and 16 mg/kg respectively) and transcardially perfused with phosphate-buffered saline (PBS), then 4% paraformaldehyde (PFA) in PBS. Brains and spinal cords were dissected and postfixed at 4° C in 4% PFA overnight and cryopreserved sequentially in 15% and 30% sucrose in PBS at 4° C before being embedded in O.C.T (Fisher Scientific). 30 μm sections were cut with a cryostat (Leica) and mounted on slides, air-dried and stored at −80° C. Alternatively, tissues were embedded in 4% low-melt agarose after postfixation, and 50- to 100-μm sections were cut with a vibratome (Leica). Embryonic spinal cords were dissected and fixed in 4% PFA in PBS for 2h and processed as described above.
Immunohistochemistry
For immunostaining, vibratome free-floating sections and cryosections were permeabilized with 0.5% Triton X-100 in PBS, blocked in 0.1% Triton X-100, 5% BSA and 5% donkey serum in PBS or in 0.2% Gelatin, 0.5% Triton X-100 in PBS for 1–2 h at room temperature and incubated with primary antibodies overnight at 4° C in blocking solution. The following primary antibodies were used: rabbit anti-Zic2 (1:1000) (gift from E. Herrera, Neuroscience Institute Alicante, Spain), rabbit anti-Pax2 (1:1000) (71–6000, Invitrogen), guinea pig anti-Lmx1B (1:10,000) (gift from C. Birchmeier, Max Delbrück Center for Molecular Medicine, Germany), rabbit anti-RORα (1:1000) (H-65, Santa Cruz), rabbit anti-Tcfap2β (1:500) (H-87, Santa Cruz), mouse anti-SATB2 (1:500) (A4B10, Abcam), rabbit anti-c-Fos (1:2000) (sc-52, Santa Cruz), rabbit monoclonal anti-c-Fos (1:750) (9F6, Cell signaling), goat anti-mCherry (1:1000) (AB0040–200, Sicgen), rabbit anti-mCherry (1:750) (PA5–34974, Invitrogen), chicken anti-GFP (1:2000) (A10262, Invitrogen), goat anti-Ret (1:300) (AF482, R&D Systems), rabbit anti-TrkA (1:500) (06–574, Millipore), rabbit anti-CGRP (1:3000) (PC205L, Calbiochem), anti-NFH (1:1000) (NA1211, Affiniti), goat anti-TrkC (1:500) (AF1404, R&D Systems), goat anti-TrkB (1:1000) (AF1494, R&D), sheep anti-TH (1:1000) (AB1542, Millipore), goat anti-ChAT 1:500 (AB144, Millipore), guinea pig anti-vGluT2 (1:2000) (AB2251, Millipore), guinea pig anti-vGluT1 (1:3000) (AB5905, Millipore), Streptavidin-488 conjugated (1:250) (S11223, Thermo Fisher). After three 30 min washes in 1 × PBS and 0.1% Triton X-100, sections were incubated with secondary antibody for 2 h at room temperature. The following secondary antibodies were used: donkey anti-rabbit/mouse/goat/chicken/guinea pig Alexa Fluor 488 or Cy2/Alexa Fluor 549 or Cy3/ Alexa Fluor 647 or Cy5 (1:800), in combination with Neurotrace 640/660 (1:500) (N21483, Thermo Fisher) in blocking solution. After three 15 min washes in 1 × PBS and 0.1% Triton X-100, sections were mounted with fluorescence mounting medium (DAKO).
Microscopy and image analysis
Fluorescence z stack images were acquired with a Leica SP8 confocal microscope and an Olympus FV1000 confocal microscope, and mosaic images were stitched using the TileScan function of LAS X software. Images were minimally processed with Fiji/ImageJ software (NIH) to enhance brightness and contrast. Median filters were used to decrease noise. For co-localization, analysis quantification was done in single confocal z sections. Cell counting was performed using the ImageJ Cell Counter plug-in. Quantification of anterograde genetically labeled PSDC axons was done as described (Grider et al., 2006). Briefly, the Hessian filter of Feature J was applied, selecting for the smallest eigen values. The resulting eigen image was converted into a binary image by applying a threshold in ImageJ. The number of pixels corresponding to positive mCherry labeling was summed for each ROI (gracile and cuneate nuclei), and the degree of innervation was expressed as a percentage of positive labeling in the areas. Finally the values obtained from the 3 anterior-posterior regions analyzed were averaged for each mouse.
Neuronal spatial analysis
The position of neurons in the spinal cord was analyzed in Fiji/ImageJ. Cartesian coordinates for each neuron were determined in the transverse spinal cord plane with respect to the midpoint of the central canal, defined as position (0,0). Coordinates were plotted using GraphPad Prism to display the position of each individual cell. Two-dimensional kernel density estimations were used to estimate the probability density functions of INs location along the x and y axes and were created in R (http://www.r-project.org) using the ‘kde2d’ function from the ‘MASS’ library. Bandwidths in the density estimation were chosen using the “bandwidth.nrd” function. Estimates were graphically displayed as contour plots, with the contour lines connecting points of equal probability density.
Pharmacological treatments
For induction of the Zic2cre, E14.5 pregnant females received a dose of tamoxifen (Sigma) by gavaging (3mg/40 g body weight). At E19.5 a caesarean delivery was performed and pups given to foster mothers. For ablation experiments, both Zic2creER;-Cdx2FlpO::DTR;Ai14 and control mice (Zic2creER::DTR;Ai14 or Cdx2FlpO::DTR;Ai14) received a double intraperitoneal (i.p.) injection of diphtheria toxin (50ng/g) (ListLabs) with 72h interval at 6 weeks post-natal. Behavior experiments started 2 weeks after the last injection. For chemogenetic behavior manipulations, both Zic2creER;CdxFlpO::hM3Dq and control mice (Zic2creER::hM3Dq or Cdx2FlpO::hM3Dq) received an i.p. injection of CNO (2 mg/kg diluted in vehicle) (Sigma) or the equivalent volume of vehicle (2% DMSO in saline). Behavior experiments started 20–30 min after injection. We observed ectopic GFP expression of the Cdx2FlpO transgene in 16% of the animals, which were thus excluded from the behavioral analysis.
Behavior assays
Animals were acclimated to the behavioral context during three days for daily 30 min sessions, prior to the day of behavioral testing.
For all experiments, the investigator performing behavioral tests was blinded to genotypes.
Dynamic brush
To assess sensitivity to dynamic light touch, we used a dynamic light brush test (Bourane et al., 2015). Mice were placed in individual small cages with a wire mesh floor and the plantar surface of the hindpaw was lightly stimulated with a soft brush (#6) by gently stroking from heel to toe. The brush stimulation was repeated 10 times on alternating left- and right-paws and the percentage of paw withdrawals from the 10 stimuli was calculated.
Static Brush
To assess sensitivity to static light touch, we used a brush test. Mice were placed in individual small cages with a wire mesh floor. A soft brush (#6) was applied perpendicularly to the plantar surface of the hindpaw. The brush stimulation was repeated 10 times on alternating left- and right-paws and the percentage of paw withdrawals from the 10 stimuli was calculated.
Von Frey
To assess sensitivity to static touch and mechanical pain, we used the von Frey test. Mice were placed in individual small cages with a wire mesh floor. Calibrated von Frey monofilaments (0.02–2 g) were used to stimulate the medial plantar surface of the hindpaws. Withdrawal, licking and biting following or immediately after the 5 s stimulus were considered as a positive response. The paw withdrawal threshold (PWT) was determined according to Yalcin et al. (2014). If the animal reacted 3 times to the same filament, this was considered a positive outcome. When two consecutive filaments got positive scores, the PWT is the lower of the two g values.
Pinprick
Mechanical pain was tested with the pinprick test. Mice were placed in individual small cages with a wire mesh floor and the plantar surface of the hindpaw was stimulated with an Austerlitz insect pin (0.02 mm; Fine Science Tools). The pin stimulation was repeated 10 times and the percentage of paw withdrawals calculated.
Dynamic Hot plate
To assess heat sensitivity, mice were acclimated to the testing apparatus with a uniformly heated aluminum floor (32° C) for 10 min on the day of the experiment and previous day (IITC Life Science, USA). During the assay, the floor temperature was set to increase from 32° C to 55° C at a rate of 3° C per minute. The withdrawal threshold was determined, and the assay stopped, when the animal licked or shook the hindpaw. The test was repeated 3 times and the temperature threshold calculated as the average temperature for paw withdrawal.
Cold plantar assay
Animals were acclimated on a small cage with a glass plate floor and a cold stimulus was applied to the hindpaw through the 3 mm thick glass using a pellet of compressed dry ice (Brenner et al., 2012). The latency to withdrawal from the cooled glass was used as a measure of the cold response threshold. The stimulation was repeated 7 times on alternating left and right paws, and the average time to withdrawal was calculated per animal.
Chloroquine induced-scratch
To test for pruritogen (itch)-induced scratching behavior, 200 μg of chloroquine (Sigma) dissolved in 50 μL of sterile saline was injected subcutaneously in the nape of the neck (Bourane et al., 2015). The animals were recorded with a webcam (Logitech C920 HD Pro) for 30 min and the scratching bouts were quantified.
Textural Place Preference
For testing textural discrimination, we developed a Place Preference assay based on Wetzel et al. (2007). Mice were placed on a two-chamber custom made Plexiglas box (two 20 cm L x 20 cm W x 25 cm H) connected by a small overture (5 cm L x 6 cm H). One of the chambers was covered with rough sand paper (30 K grit) and the other chamber with fine sand paper (150 K grit). Test sessions were conducted for 15 min in the dark under infrared illumination to remove visual cues and recorded with an infrared webcam (Microsoft LifeCam HD-3000). After 15 min of exploration, mice were returned to the home cage and the test was repeated after changing the sand paper and inverting the location of the rough and fine sand paper chambers. On a different day, we repeated the test using 80 K (intermediate) grit sand paper (instead of 30 K) on the floor of one the chambers, maintaining the fine sand paper (150 K) on the other chamber. Animal location was tracked, and the time spent in each chamber was assessed with Ethovision XT 11 software (Noldus). Preference indices (P.I.) were calculated as average of [(Time in rough chamber Time in fine chamber)/Total time].
Rotarod
The accelerating rotarod (Ugo Basile, Italy) was used to test gross motor ability and coordination. Mice were trained on two consecutive days. Training consisted of mice being placed on the rotarod moving at a constant speed of 5 rpm for 5 min. Mice were trained to stay on the rotarod for the entire 5 min. Mice were tested by accelerating the rotarod from 5 rpm to 40 rpm in 5 min. Each animal was subject to three trials, with an interval of 15 min between each trial. The latency to fall was averaged for the three trials.
Open field
To test overall locomotor activity, mice were allowed to explore a custom made Plexiglas arena (40 cm × 3 40 cm W x 25 cm H) for 10 min. Animal location was tracked, and the number of entries to the center of the arena (20 cm × 20 cm square), velocity, and distance traveled were assessed with Ethovision XT 11 software (Noldus).
Elevated Beam
Fine motor coordination was evaluated using the elevated beam test. Mice were tested on different diameter beams (1 m long, 50 cm high). Training on the first day consisted of 4 trials on a 23 mm wood round beam, and on the second day of 1 trial on a 23 mm wood round beam and 3 trials on a 12 mm riffled wood round beam. On the test day, mice were video recorded with a Grasshoper3 GS3-U3–51S5M high speed camera (Point Grey Research) at 100 fps on three consecutive runs/trials, starting with the 12 mm wood beam followed by a 10 mm metal square beam and a 5 mm metal square beam. Side and bottom views of the animal (mirror and direct view) were used to analyze the number of foot slips. The analysis of foot slips was done manually by an experimenter blind to the genotypes.
Descending Beam
Mice were trained to descend a 10 × 10 mm metal beam inclined at 18°. On the consecutive day, video recordings were taken on three consecutive trials, starting with the 10 ×10 metal beam, followed by the 5 × 10 mm metal beam. The analysis of foot slips was done manually by an experimenter blind to the genotypes. Mice that did not walk with the hindlimbs on the beam were excluded from this analysis.
Treadmill
Gait analysis was performed using the TreadScan apparatus (CleverSys, Reston, VA). Mice were trained at a treadmill speed of 9 cm/ s, during 10 min on two consecutive days. Recordings at 63 frames/s for 30 s were acquired with the TreadScan software. Segments where the animal displayed continuous locomotion were automatically selected and analyzed using the TreadScan software. Gait analysis was performed at 9 cm/s. The gait parameters of stride, stance, and swing time were automatically calculated and average values for each hindlimb and trial were used for statistical analysis. The limb coordination parameters (phase coupling) were automatically calculated, averaging the values for each hindlimb and trial for the homologous coupling, and all four limbs for the homolateral and diagonal coupling.
Conditioned Place Aversion (CPA)
To test for the valence of Zic2 chemogenetic activation we modified a conditioned place aversion test described in Vrontou et al. (2013). A custom made plexiglas 2-chamber arena (two 20 cm L x 20 cm W x 30 cm H, connected by a closable door 5 cm x 5 cm) with different wall patterns and different floor grids was housed inside a soundproof chamber equipped with houselights (TSE Multiconditioning System). On day 1, each mouse was placed in the left compartment and allowed to explore the entire apparatus freely for 30 min (Pre-Test). After the pre-test the initial preference of each mouse for a given side compartment was recorded. With our apparatus design, most of the mice showed an initial preference for one of the compartments. Conditioning was initiated on day 2 and encompassed four sessions performed on four consecutive days. In the first conditioning session, mice were injected i.p. with CNO (2mg/kg) and placed for 1 h in the initially preferred (I.P.) compartment. On day 3, during the second conditioning session, mice were injected with vehicle (2% DMSO) and confined for 1h in the opposite (that is, initially non-preferred, I.N.P.) compartment. On day 4 and day 5 the first and second conditioning sessions were repeated, respectively. On day 6, the mice were tested for their side compartment preference by placing them in the left compartment and allowing them to explore the entire apparatus freely for 30min (post-test). Animal location was tracked, and the time spent in each box was assessed with Ethovision XT 11 software (Noldus). Preference indices (P.I.) were calculated as [(Time in Initially Preferred (I.P.) chamber Time in Initially Non-Preferred (I.N.P.) chamber)/Total time].
Stereotaxic surgeries
6 to 8 weeks old mice were anesthetized with isofluorane (1.5%) and placed in a stereotaxic frame (Kopf Instruments). Body temperature was maintained with a heating pad. The analgesics Metamizol (0.2 mg/g bodyweight, oral) and Carprofen (5mg/kg bodyweight, subcutaneous) were administrated.
For retrograde intersectional experiments, mice were injected bilaterally using a fine glass capillary with ~250 nL of virus in the DCN by using the following coordinates with respect to the bregma: −7.68 mm anteroposterior, ± 1 mm lateral, −2.26 mm ventral (from dura). In the same surgery, mice used in double retrograde/anterograde tracing experiments were also injected unilaterally in either GRN ( −6.64 mm anteroposterior, +0.44 mm lateral, −5 mm ventral), or LVN ( −6 mm anteroposterior, +1.35 mm lateral, −3.3 mm ventral). For CST tracings, the motor cortex was injected with the anterograde tracer biotin dextran amine (10% BDA 10000) in total 0.8 μl into eight injection sites (in an area spanning +2/ −1 mm anteroposterior and +1/+2.5 mm lateral, at −0.3 mm and −0.8 mm ventral). At 15 days after surgery, mice were perfused, and spinal cords were processed for histology. Viral injection location and expression was subjected to post hoc analysis, and mice that didn’t fulfill the criteria were excluded from the behavioral analysis.
Rabies virus tracings
P7 animals were anesthetized with isofluorane (2%) and placed on a heating pad. After incision of the skin, a laminectomy was performed at the C2 or L1/L2 levels. The dura matter was carefully perforated with a fine needle to expose the spinal cord. ~0.25–0.35 μL of virus were injected unilaterally on the dorsal spinal cord −300 to 500 μm ventral with a fine glass capillary. The skin was then closed using a Reflex 7 skin closure system and tissue adhesive (3M Vetbond). Animals were perfused 7 days post-infection.
Motor neuron tracings
Motor neurons were retrogradely labeled in P1 animals via intramuscular injection of ~0.5 μL of Cholera toxin subunit B (CTB), Alexa Fluor 555 conjugate (2 mg/ml) (Invitrogen) into the quadriceps muscles with a fine glass capillary through the skin. Animals were sacrificed and spinal cords processed 3 h later.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics
No statistical methods were used to predetermine sample sizes. Data presented as bar graphs indicate mean ± standard error of the mean (SEM). Dots in bar graphs represent individual values per mouse. Pairwise comparisons were calculated with unpaired or paired two-tailed t tests. Statistical analyses were performed in GraphPad Prism. Statistical details of experiments can be found in the figure legends or main text. Significance levels are indicated as follows: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
DATA AND CODE AVAILABILITY
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
All custom-written R codes used in this study are available from the corresponding author.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal Pax2 (1:1000) | Thermo Fisher Scientific | Cat#71-6000; RRID:AB_2533990 |
| Guinea pig polyclonal Lmx1B (1:10000) | C. Birchmeier (MDC, Berlin) | N/A |
| Rabbit polyclonal Zic2 (1:1000) | E. Herrera (Neuroscience Institute Alicante) | N/A |
| Rabbit polyclonal RORα (H-65) (1:1000) | Santa Cruz Biotechnology | Cat# sc-28612; RRID:AB_2180141 |
| Rabbit polyclonal AP-2β (H87) (1:500) | Santa Cruz Biotechnology | Cat# sc-8976; RRID:AB_633936 |
| Mouse monoclonal SATB2 (SATBA4B10) (1:500) | Abcam | Cat# ab51502; RRID:AB_882455 |
| Rabbit polyclonal c-Fos (1:2000) | Santa Cruz Biotechnology | Cat# sc-52; RRID:AB_2106783 |
| Rabbit monoclonal c-Fos (9F6) (1:750) | Cell Signaling Technology | Cat# 2250; RRID:AB_2247211 |
| Goat polyclonal mCherry (1:1000) | SICGEN | Cat# AB0040-200; RRID:AB_2333092 |
| Rabbit polyclonal mCherry (1:750) | Thermo Fisher Scientific | Cat# PA5-34974, RRID:AB_2552323 |
| Chicken polyclonal GFP tag (1:2000) | Thermo Fisher Scientific | Cat# A10262; RRID:AB_2534023 |
| Goat polyclonal Ret (1:300) | R&D Systems | Cat# AF482; RRID:AB_2301030 |
| Rabbit polyclonal TrkA (1:500) | Millipore | Cat# 06-574; RRID:AB_310180 |
| Rabbit polyclonal CGRP (1:3000) | Millipore | Cat# PC205L; RRID:AB_2068524 |
| Rabbit polyclonal Neurofilament H (1:1000) | Enzo Life Sciences | Cat# BML-NA1211; RRID:AB_10540573 |
| Goat polyclonal TrkC (1:500) | R&D Systems | Cat# AF1404; RRID:AB_2155412 |
| Goat polyclonal ChAT (1:500) | Millipore | Cat# AB144; RRID:AB_11212843 |
| Guinea pig polyclonal vGluT2 (1:2000) | Millipore | Cat# AB2251; RRID:AB_1587626 |
| Guinea pig polyclonal vGluT1 (1:3000) | Millipore | Cat# AB5905; RRID:AB_2301751 |
| Sheep polyclonal TH (1:1000) | Millipore | Cat# AB1542; RRID:AB_90755 |
| Goat polyclonal TrkB (1:1000) | R&D Systems | Cat# AF1494; RRID:AB_2155264 |
| Bacterial and Virus Strains | ||
| EnvA ΔG rabies-eGFP | Viral Vector Core, Salk Institute (Osakada et al., 2011) | Addgene#32635; RRID:Addgene_32635 |
| rAAVretro-Ef1a-FlpO | Viral Vector Core, Salk Institute (Tervo et al., 2016) | Addgene#55637; RRID:Addgene_55637 |
| AAV5-hSyn-EYFP | UNC Vector Core (Warden et al., 2012) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Tamoxifen | Sigma-Aldrich | Cat# T5648; CAS:10540-29-1 |
| Chloroquine diphosphate salt | Sigma-Aldrich | Cat# C6628; CAS:50-63-5 |
| Clozapine N-oxide | Sigma-Aldrich | Cat# C0832; CAS:34233-69-7 |
| Diphtheria Toxin, Unnicked, from Corynebacterium diphtheriae | List Biological Laboratories | Cat# 150 |
| Biotinylated Dextran Amine | Thermo Fisher Scientific | Cat# D1956 |
| Streptavidin, Alexa Fluor 488 conjugate | Thermo Fisher Scientific | Cat# S11223 |
| NeuroTrace 640/660 | Thermo Fisher Scientific | Cat# N21483; RRID:AB_2572212 |
| Cholera Toxin Subunit B (Recombinant), Alexa Fluor 555 Conjugate | Thermo Fisher Scientific | Cat# C34776 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Zic2creER | This paper | N/A |
| Mouse: Zic2creERbroad | This paper | N/A |
| Mouse: Taulox-stop-lox-SynGFP | Tripodi et al., 2011 | N/A |
| Mouse: RC::FL-hM3Dq | Sciolino et al., 2016 | Cat# JAX:026942; RRID:IMSR_JAX:026942 |
| Mouse: Tauds-DTR | Duan et al., 2014 | N/A |
| Mouse: Cdx2-FlpO | Britz et al., 2015 | N/A |
| Mouse: Ai9-tdTomato | The Jackson Laboratory | Cat# JAX:007909; RRID:IMSR_JAX:007909 |
| Mouse: Ai14-tdTomato | The Jackson Laboratory | Cat# JAX:007914; RRID:IMSR_JAX:007914 |
| Mouse: Ai65D-tdTomato | The Jackson Laboratory | Cat# JAX:021875; RRID:IMSR_JAX:021875 |
| Mouse: R26ΦC31 | The Jackson Laboratory | Cat# JAX:007670; RRID:IMSR_JAX:007670 |
| Mouse: RΦT | Takatoh et al., 2013 | Cat# JAX:024708; RRID:IMSR_JAX:024708 |
| Software and Algorithms | ||
| Software: LAS X version 3.5 | Leica Microsystems | https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/ |
| TreadScan Version 3.00 | CleverSystems | N/A |
| Ethovision XT 11 | Noldus | N/A |
| Prism 5 | GraphPad | https://www.graphpad.com |
| Fiji (ImageJ) | Schindelin et al., 2012 | https://imagej.net/Fiji |
| ImageJ Cell Counter Plugin | Kurt de Vos, Univ. of Sheffield, UK | https://imagej.nih.gov/ij/plugins/cell-counter.html |
| ImageJ FeatureJ Plugin | Eric Meijering. Univ. Med. Center Rotterdam, 2003 | https://imagescience.org/meijering/software/featurej/ |
| R: kde2D function | The R foundation | https://www.r-project.org |
| Allen Brain Explorer | Lau et al., 2008 | http://portal.brain-map.org/ |
Highlights.
Excitatory spinal Zic2 neurons transmit light touch information
Spinal Zic2 neurons are required for corrective motor movements
Zic2 neurons contribute to the post-synaptic dorsal column projection (PSDC)
Zic2 PSDCs integrate sensory cutaneous input with descending motor information
ACKNOWLEDGMENTS
We thank the MPI transgenic service unit for their technical assistance; Patricia Jensen (NIEHS), Silvia Arber (FMI), Benjamin Arenkiel (Baylor College of Medicine), and Jan Deussing (MPI of Psychiatry) for mouse lines; Eloisa Herrera (IN Alicante) and Carmen Birchmeier (MDC) for antibodies; Augusto Escalante for screening transgenic lines; Marion Ponserre for R scripts; and Graziana Gatto for constructive discussions. This study was supported by the Max-Planck Society and the Deutsche Forschungsgemeinschaft (SPP1665) to R.K and the National Institutes of Health (NS111643) to M.G.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.neuron.2019.08.029.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Abraira VE, and Ginty DD (2013). The sensory neurons of touch. Neuron 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, et al. (2017). The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 168, 295–310.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Megat S, Barthas F, Waltisperger E, Kremer M, Salvat E, and Barrot M. (2014). The Sciatic Nerve Cuffing Model of Neuropathic Pain in Mice. J. Vis. Exp. 89, e51608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaldella E, Takeoka A, Sigrist M, and Arber S. (2015). Multisensory Signaling Shapes Vestibulo-Motor Circuit Specificity. Cell 163, 301–312. [DOI] [PubMed] [Google Scholar]
- Bourane S, Grossmann KS, Britz O, Dalet A, Del Barrio MG, Stam FJ, Garcia-Campmany L, Koch S, and Goulding M. (2015). Identification of a spinal circuit for light touch and fine motor control. Cell 160, 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, and Kiehn O. (2015). Descending Command Neurons in the Brainstem that Halt Locomotion. Cell 163, 1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer LJG, and Rossignol S. (2003). Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J. Neurophysiol. 90, 3625–3639. [DOI] [PubMed] [Google Scholar]
- Brenner DS, Golden JP, and Gereau RWIV IV (2012). A novel behavioral assay for measuring cold sensation in mice. PLoS One 7, e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretzner F, and Drew T. (2005). Motor cortical modulation of cutaneous reflex responses in the hindlimb of the intact cat. J. Neurophysiol. 94, 673–687. [DOI] [PubMed] [Google Scholar]
- Britz O, Zhang J, Grossmann KS, Dyck J, Kim JC, Dymecki S, Gosgnach S, and Goulding M. (2015). A genetically defined asymmetry underlies the inhibitory control of flexor–extensor locomotor movements. eLife 4, e04718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, and Fyffe RE (1981). Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J. Physiol. 321, 31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, and Chopek JW (2018). Reticulospinal Systems for Tuning Motor Commands. Front. Neural Circuits 12, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, and Brownstone RM (2013). Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron 78, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano V, Leiras R, Goñi-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G, and Kiehn O. (2018). Midbrain circuits that set locomotor speed and gait selection. Nature 553, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli P, Pivetta C, Soledad Esposito M, and Arber S. (2017). Locomotor speed control circuits in the caudal brainstem. Nature 551, 373–377. [DOI] [PubMed] [Google Scholar]
- Chaveroche MK, Ghigo JM, and d’Enfert C. (2000). A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 28, E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, et al. (2004). Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat. Neurosci. 7, 510–517. [DOI] [PubMed] [Google Scholar]
- Cheng L, Duan B, Huang T, Zhang Y, Chen Y, Britz O, Garcia-Campmany L, Ren X, Vong L, Lowell BB, et al. (2017). Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat. Neurosci. 20, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Miao X, Liang L, Abdus-Saboor I, Olson W, Fleming MS, Ma M, Tao YX, and Luo W. (2016). Identification of Early RET+ Deep Dorsal Spinal Cord Interneurons in Gating Pain. Neuron 91, 1137–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y-Q, Yin J, Kania A, Zhao Z-Q, Johnson RL, and Chen Z-F (2004). Lmx1b controls the differentiation and migration of the superficial dorsal horn neurons of the spinal cord. Development 131, 3693–3703. [DOI] [PubMed] [Google Scholar]
- Dobry PJ, and Casey KL (1972). Roughness discrimination in cats with dorsal column lesions. Brain Res. 44, 385–397. [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, et al. (2014). Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enevoldson TP, and Gordon G. (1989). Postsynaptic dorsal column neurons in the cat: a study with retrograde transport of horseradish peroxidase. Exp. Brain Res. 75, 611–620. [DOI] [PubMed] [Google Scholar]
- Escalante A, Murillo B, Morenilla-Palao C, Klar A, and Herrera E. (2013). Zic2-dependent axon midline avoidance controls the formation of major ipsilateral tracts in the CNS. Neuron 80, 1392–1406. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Capelli P, and Arber S. (2014). Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature 508, 351–356. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, and Chambon P. (1997). Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752–757. [DOI] [PubMed] [Google Scholar]
- Ferreira-Pinto MJ, Ruder L, Capelli P, and Arber S. (2018). Connecting Circuits for Supraspinal Control of Locomotion. Neuron 100, 361–374. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Mitchell R, Hope PJ, Molony V, and Iggo A. (1985). An a 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Res. 334, 243–254. [DOI] [PubMed] [Google Scholar]
- Glasgow SM, Henke RM, Macdonald RJ, Wright CVE, and Johnson JE (2005). Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development 132, 5461–5469. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, et al. (2017). Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider MH, Chen Q, and Shine HD (2006). Semi-automated quantification of axonal densities in labeled CNS tissue. J. Neurosci. Methods 155, 172–179. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, and Perl ER (2002). Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J. Physiol. 540, 189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilde KL, Levine AJ, Hinckley CA, Hayashi M, Montgomery JM, Gullo M, Driscoll SP, Grosschedl R, Kohwi Y, Kohwi-Shigematsu T, and Pfaff SL (2016). Satb2 Is Required for the Development of a Spinal Exteroceptive Microcircuit that Modulates Limb Position. Neuron 91, 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Hedén C, Szabo Läckberg Z, and Yin X-K (1997). Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur. J. Neurosci. 9, 1375–1387. [DOI] [PubMed] [Google Scholar]
- Johansson T, Broll I, Frenz T, Hemmers S, Becher B, Zeilhofer HU, and Buch T. (2010). Building a zoo of mice for genetic analyses: a comprehensive protocol for the rapid generation of BAC transgenic mice. Genesis 48, 264–280. [DOI] [PubMed] [Google Scholar]
- Koch SC, Del Barrio MG, Dalet A, Gatto G, Günther T, Zhang J, Seidler B, Saur D, Schüle R, and Goulding M. (2017). RORβ Spinal Interneurons Gate Sensory Transmission during Locomotion to Secure a Fluid Walking Gait. Neuron 96, 1419–1431.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SC, Acton D, and Goulding M. (2018). Spinal Circuits for Touch, Pain, and Itch. Annu. Rev. Physiol. 80, 189–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemend F, and Ernfors P. (2012). Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 35, 373–381. [DOI] [PubMed] [Google Scholar]
- Lau C, Ng L, Thompson C, Pathak S, Kuan L, Jones A, and Hawrylycz M. (2008). Exploration and visualization of gene expression with neuroanatomy in the adult mouse brain. BMC Bioinformatics 9, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Hinckley CA, Hilde KL, Driscoll SP, Poon TH, Montgomery JM, and Pfaff SL (2014). Identification of a cellular node for motor control pathways. Nat. Neurosci. 17, 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinsson A, Holmberg H, Broman J, Zhang M, and Schouenborg J. (2002). Spinal sensorimotor transformation: relation between cutaneous somatotopy and a reflex network. J. Neurosci. 22, 8170–8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Paxinos G, and Watson C. (2012a). The red nucleus and the rubrospinal projection in the mouse. Brain Struct. Funct. 217, 221–232. [DOI] [PubMed] [Google Scholar]
- Liang H, Paxinos G, and Watson C. (2012b). Spinal projections from the presumptive midbrain locomotor region in the mouse. Brain Struct. Funct. 217, 211–219. [DOI] [PubMed] [Google Scholar]
- Liang H, Watson C, and Paxinos G. (2016). Terminations of reticulospinal fibers originating from the gigantocellular reticular formation in the mouse spinal cord. Brain Struct. Funct. 221, 1623–1633. [DOI] [PubMed] [Google Scholar]
- Liu Y, Latremoliere A, Li X, Zhang Z, Chen M, Wang X, Fang C, Zhu J, Alexandre C, Gao Z, et al. (2018). Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, and Ginty DD (2009). Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron 64, 841–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. (2010). Labeled lines meet and talk: population coding of somatic sensations. J. Clin. Invest. 120, 3773–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, et al. (2015). Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring F, Halder P, Seal RP, and Stucky CL (2018). Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 100, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, and Snider WD (1995). Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J. Comp. Neurol. 361, 404–416. [DOI] [PubMed] [Google Scholar]
- Moreno-López Y, Olivares-Moreno R, Cordero-Erausquin M, and Rojas-Piloni G. (2016). Sensorimotor Integration by Corticospinal System. Front. Neuroanat. 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Croce K, Belton T, Akay T, and Jessell TM (2018). Balance Control Mediated by Vestibular Circuits Directing Limb Extension or Antagonist Muscle Co-activation. Cell Rep. 22, 1325–1338. [DOI] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, and Callaway EM (2011). New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paixão S, Balijepalli A, Serradj N, Niu J, Luo W, Martin JH, and Klein R. (2013). EphrinB3/EphA4-mediated guidance of ascending and descending spinal tracts. Neuron 80, 1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek I, Bui T, Wright AT, and Brownstone RM (2014). Cutaneous afferent regulation of motor function. Acta Neurobiol. Exp. (Warsz.) 74, 158–171. [DOI] [PubMed] [Google Scholar]
- Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, and Seal RP (2015). Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 87, 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan L-Y, Ribeiro-da-Silva A, et al. (2015). Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 13, 1246–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, and Soriano P. (2007). High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS One 2, e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, and Gossard JP (2006). Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 86, 89–154. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouenborg J, Weng HR, Kalliomäki J, and Holmberg H. (1995). A survey of spinal dorsal horn neurones encoding the spatial organization of withdrawal reflexes in the rat. Exp. Brain Res. 106, 19–27. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Plummer NW, Chen YW, Alexander GM, Robertson SD, Dudek SM, McElligott ZA, and Jensen P. (2016). Recombinase-Dependent Mouse Lines for Chemogenetic Activation of Genetically Defined Cell Types. Cell Rep. 15, 2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatoh J, Nelson A, Zhou X, Bolton MM, Ehlers MD, Arenkiel BR, Mooney R, and Wang F. (2013). New modules are added to vibrissal premotor circuitry with the emergence of exploratory whisking. Neuron 77, 346–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, et al. (2016). A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 92, 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi M, Stepien AE, and Arber S. (2011). Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature 479, 61–66. [DOI] [PubMed] [Google Scholar]
- Ueno M, Nakamura Y, Li J, Gu Z, Niehaus J, Maezawa M, Crone SA, Goulding M, Baccei ML, and Yoshida Y. (2018). Corticospinal Circuits from the Sensory and Motor Cortices Differentially Regulate Skilled Movements through Distinct Spinal Interneurons. Cell Rep. 23, 1286–1300.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrontou S, Wong AM, Rau KK, Koerber HR, and Anderson DJ (2013). Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature 493, 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim S-Y, Adhikari A, Tye KM, Frank LM, and Deisseroth K. (2012). A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, et al. (2007). A stomatin-domain protein essential for touch sensation in the mouse. Nature 445, 206–209. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann K-K, Young JAT, and Callaway EM (2007). Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Hughes DI, Riddell JS, and Todd AJ (2010). Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain 151, 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, and Stein RB (1999). What functions do reflexes serve during human locomotion? Prog. Neurobiol. 58, 185–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
All custom-written R codes used in this study are available from the corresponding author.
The data that support the findings of this study are available from the corresponding author upon reasonable request.