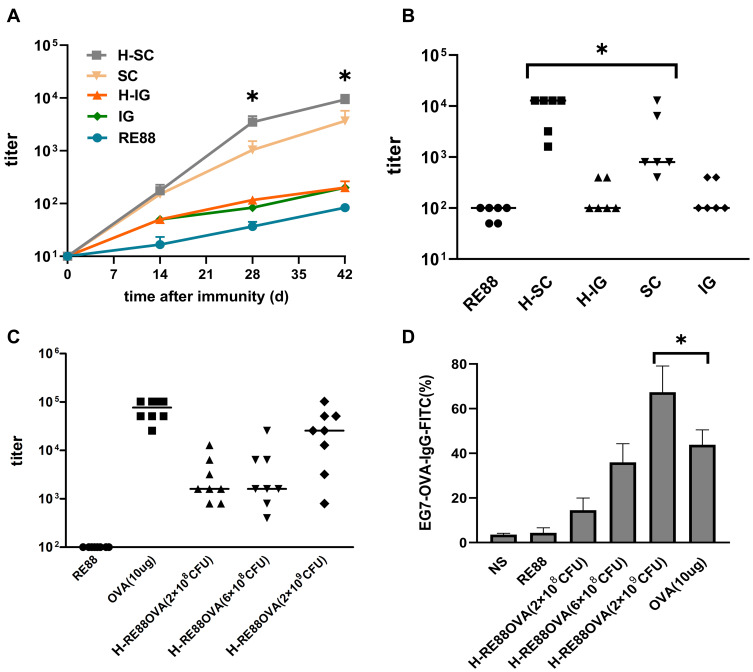
Figure 3.
Evaluation of humoral immunity induced by prophylactic vaccination in a mouse tumor model. (A) Anti-OVA IgG titer in mouse serum at various times after the first immunization (109 CFU/per mouse). Data are presented as the mean ± SEM of ≥6 independent experiments. *P < 0.05 as compared with the H-SC, H-IG groups. H-SC: subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA; SC: subcutaneous administration of non-inactivated RE88-pVLT33-OVA. H-IG: intragastric administration of H2O2-inactivated RE88-pVLT33-OVA; IG: intragastric administration of non-inactivated RE88-pVLT33-OVA; RE88: unmodified strain. (B and C) Anti-OVA IgG titer in mouse serum 42 days after the first immunization. H-RE88OVA: subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA (3 doses); OVA-10 µg (positive control), *P < 0.05. (D) Ability of the specific IgG antibody in mouse serum to bind to the target tumor cells, subcutaneous administration with various vaccine types (1:400 serum dilution) of H2O2-inactivated RE88-pVLT33-OVA (n ≥ 3).*P < 0.05.
Abbreviation: NS, normal saline (negative control).