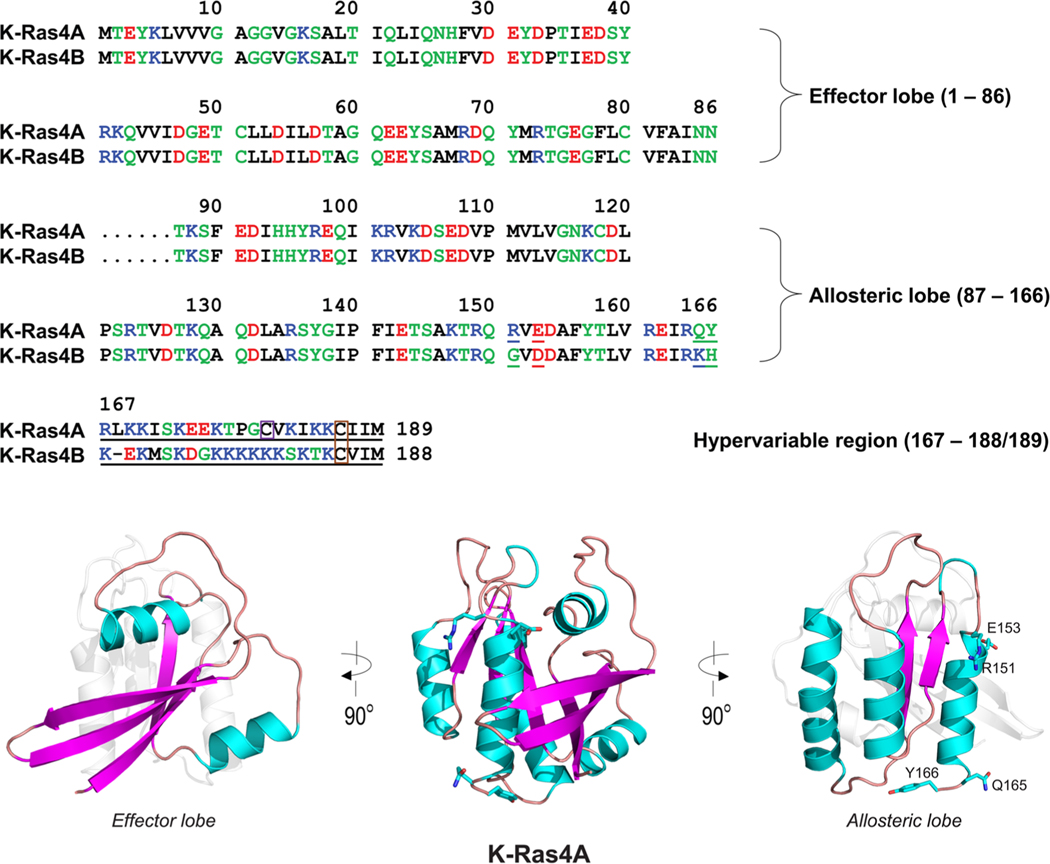
Figure 1.
Multiple sequence alignment of the amino acids in the K-Ras4A and K-Ras4B proteins. In the sequence, hydrophobic, polar/glycine, positively charged, and negatively charged residues are colored black, green, blue, and red, respectively. The nonidentity of residues in the alignment is indicated by underlined text. In the hypervariable region (HVR) sequences, a purple box denotes the palmitoylated cysteine in K-Ras4A, and an orange-box indicates the farnesylated cysteines in both K-Ras4A and K-Ras4B. The catalytic domain structures of K-Ras4A with highlighted effector lobe (left) and allosteric lobe (right) are shown. Four residues designated for the K-Ras4A catalytic domain are marked.