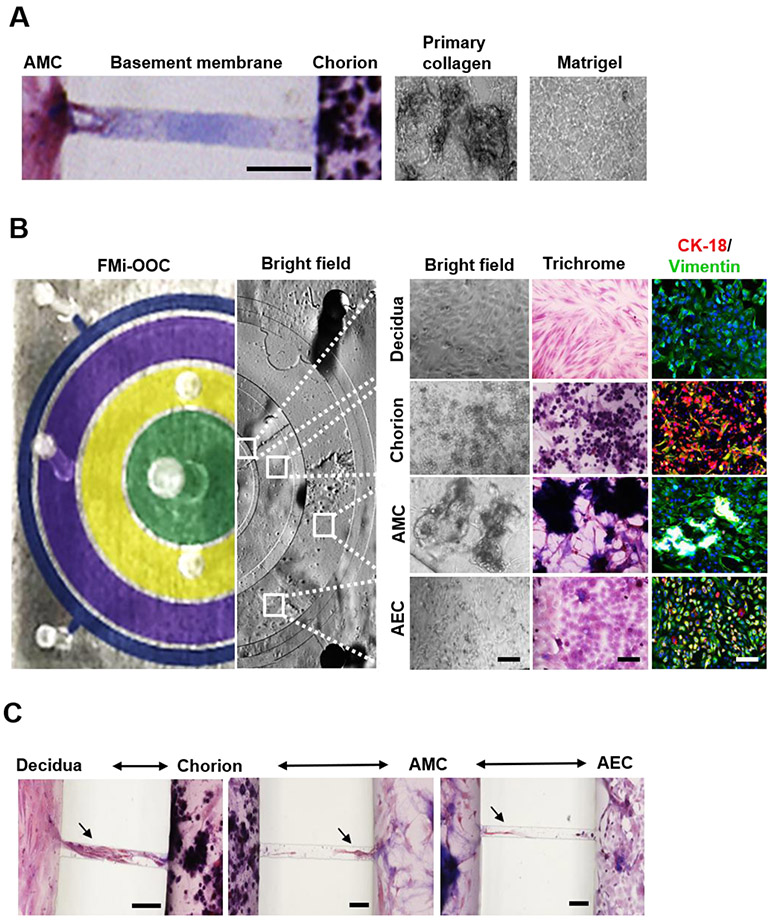
Figure 3: Characterization of decidua and amniochorionic derived cells cultured within the FMi-OOC.
A) Microchannels between the AEC and AMC, as well as the AMC and CTs/CMCs, chambers are filled with type IV collagen to recreate the two basement membranes. Collagen was stained with Masson trichome for visualization (blue color, left image). Scale bar = 100 μm. Additionally, AMCs and CTs/CMCs were cultured with decellularized primary collagen (middle image) harvested from the membrane of the amnion to provide biochemical factors necessary to recreate stromal layers, as well as function as and matrix to provide a scaffold for the cells to grow in 3D (right image).
B) On the left is a cross-sectional view of the four-chamber FMi-OOC device, highlighting the decidua chamber as green, the chorion chamber as yellow, the AMC chamber as purple, and the AEC chamber as blue. The right section of this image shows bright field microscopy images of cells growing in each cell culture chamber. A variety of in utero characteristics were measured to determine if cells grown within the FMi-OOC retained their in vivo characteristics (n = 3). These measurements included cell morphology, collagen production (Masson trichome staining [non-collagen producing cells show up as red, collagen-producing cells show up as purple, and collagen components show up as dark purple/blue]), cellular transition status (epithelial [CK-18; red] and mesenchymal [vimentin; green]) intermediate filament expression, and migratory potential. Scale bar = 50 μm.
C) Visualization of cell migration between compartments through the use of Masson trichome stain as a counter stain. Cellular migration between the chorion and amnion chambers were observed (see black arrowhead), as well as degradation of type IV collagen in the microchannel within 72 h. Scale bar = 100 μm.