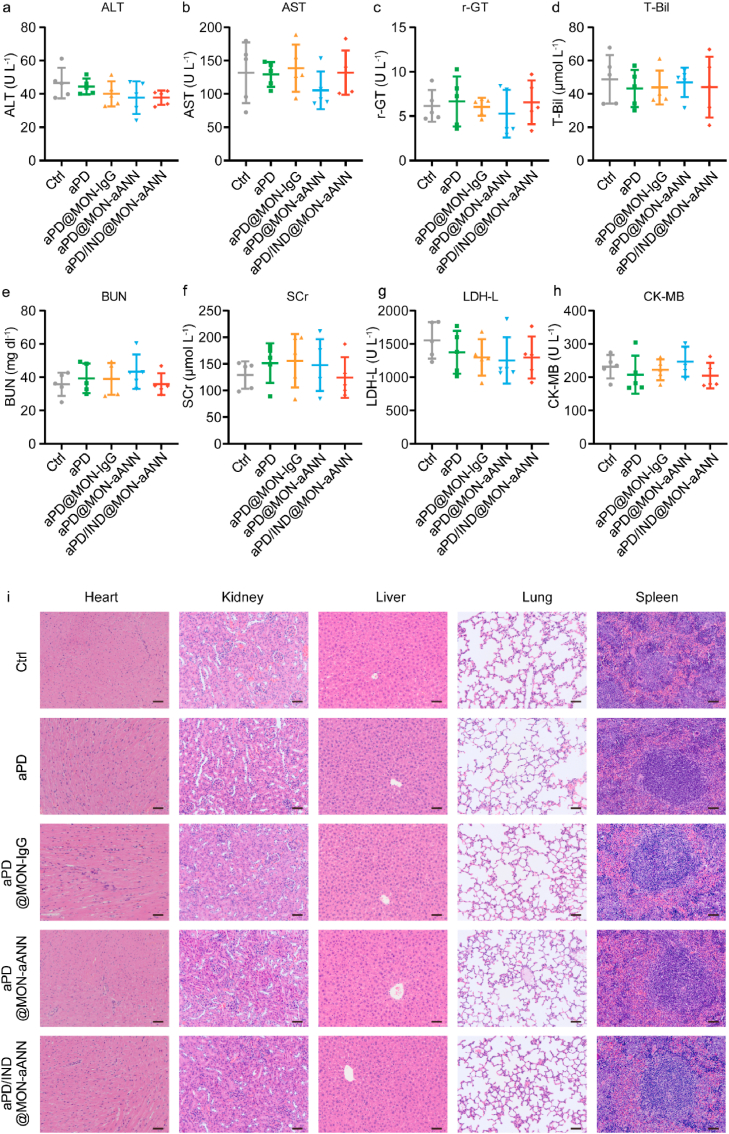
Fig. 7.
Biocompatibility and safety evaluation. (a-h) The level of ALT (a), AST (b), r-GT (c), T-Bil (d), BUN (e), SCr (f), LDH-L (g) and CK-MB (h) in the serum from subcutaneous tumor-bearing mice at the endpoint of therapeutic study (n = 5, data presented as mean ± s.d). (i) Representative H&E-stained sections of heart, kidney, liver, lung and spleen from mice of different groups. Scale bar = 50 μm.