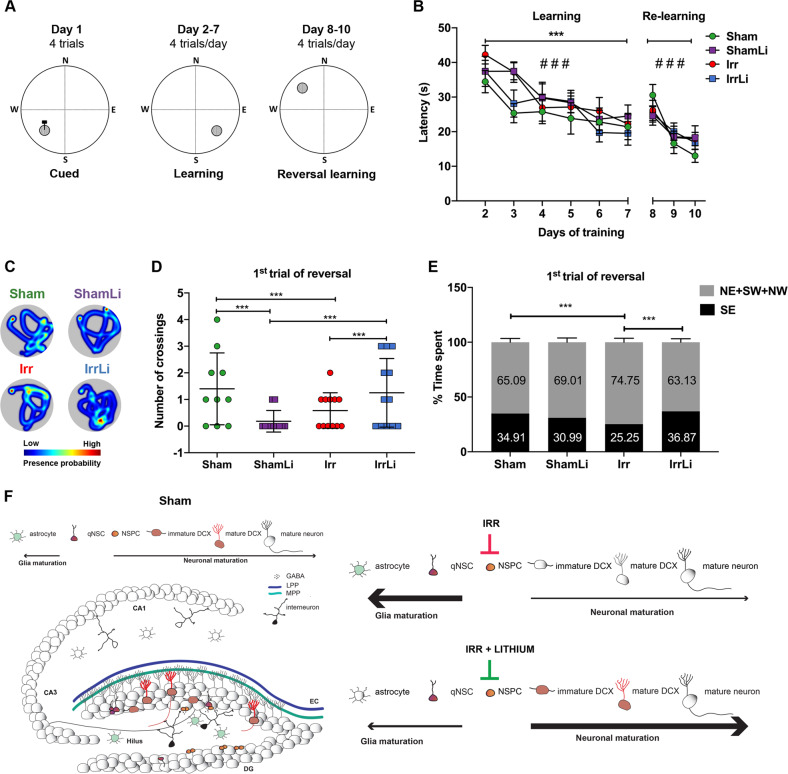
Fig. 5.
Lithium rescued the irradiation-induced deficits on hippocampal-dependent learning and memory. a Experimental protocol of the Morris water maze task; the platform was moved between the cued, learning, and reversal learning training sessions. b All groups successfully learned to navigate to the hidden goal over time in both learning (GEE for latency: W5,270 = 64.960, ###p < 0.001) and reversal learning test (GEE for latency: W2,135 = 37.8941, ###p < 0.001), but the Sham and IrrLi mice found the hidden platform faster than the Irr and ShamLi mice during the first acquisition period (days 2–7) (GEE group effect: W3,270 = 29.384, ***p < 0.001; post hoc tests: Sham vs. ShamLi p = 0.004, Sham vs. Irr p < 0.001, IrrLi vs. ShamLi p = 0.005, IrrLi vs. Irr p < 0.001). c Representative heat maps for all groups of animals of the first trial of the reversal learning. d Number of crossings over the previous goal position. The IrrLi and Sham mice showed higher preference for the previous position of the platform compared with the Irr and ShamLi mice (GLM group effect: W3,45 = 58.844, p < 0.001; post hoc tests: Sham vs. ShamLi ***p < 0.001, Sham vs. Irr ***p < 0.001, ShamLi vs. IrrLi ***p < 0.001, Irr vs. IrrLi ***p = 0.001). e Time spent in the quadrants during the first trial of the first day of reversal learning. There was a significant group effect (GLM: W3,45 = 23.068, p < 0.001). Sham and IrrLi animals exhibited a stronger preference than the Irr mice for the quadrant where the platform was located during the acquisition period (post hoc tests: Sham vs. Irr ***p < 0.001, Irr vs. IrrLi ***p = 0.001), while ShamLi had an intermediate value compared with the three other groups. f Schematic drawing of the hippocampal network on the left. Input signals from the entorhinal cortex (EC) are carried through two connectional routes made of the axons of the medial (light green) and lateral (blue) perforant pathways, MPP and LPP respectively. These axons establish stable synapses with the dendrites of the mature granule cell neurons (gray) and weak ones with the immature doublecortin (DCX) cells (red) in the granule cell layer (GCL). The subgranular zone (SGZ) is located at the boundary of the GCL and the hilus, where quiescent neural stem cells (qNSC) give rise to amplifying neural progenitors (ANP) allowing the continuous neuronal re-population of the dentate gyrus (DG). The qNSC and ANPs are multipotent stem cells, capable of giving rise to astrocytes, oligodendrocytes and neurons. The input signal from the DG is relayed to the proximal Cornus Ammonis region (CA3) through the axons of the mature granule cells that form the mossy fiber projection. The signal transduction continues to the CA1 region through the Schaffer collateral fibers and ultimately to further cortical areas. Parvalbumin (PV) interneurons in the hilus are important in modulating, through feedback and feedforward inhibition, the input signals and NSC proliferation and integration through the release of the neurotransmitter gamma-aminobutyric acid (GABA). Right top: a schematic representation of the effects of irradiation on DG. The number of astrocytes is increased while the number of ANPs is decreased. The neuronal differentiation process is decreased in favor of an astrocytic fate progression. Right bottom: a schematic representation of the effects of lithium on the irradiated DG. Lithium acts by increasing ANPs cell number and promoting neuronal fate progression as compared with astrocytic differentiation