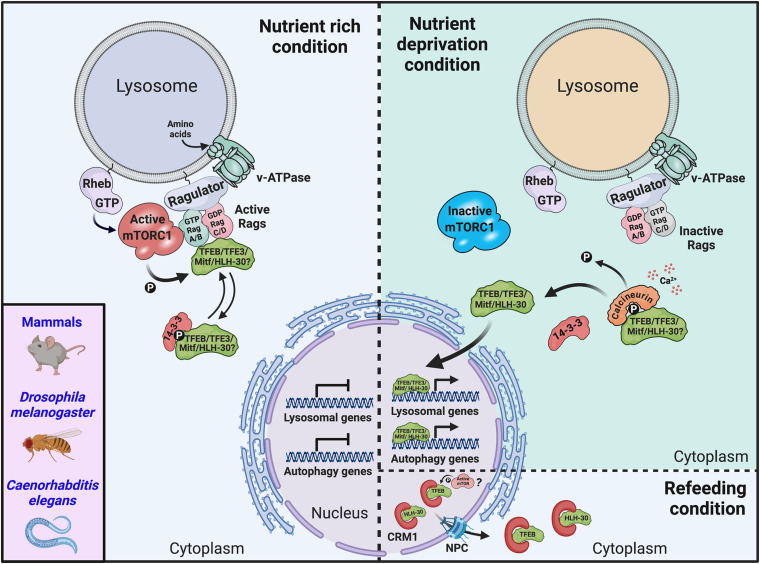
FIGURE 3.
MiT/TFE activation is regulated in response to nutrient deprivation. Schematic representation of the mechanism of MiT/TFE transcription factor regulation by nutrient levels in the cell. In nutrient-rich condition, MiT/TFE proteins mammalian target of rapamycin complex 1 (mTORC1) are recruited to the lysosomal membrane through binding to active RagGTPases. Active mTORC1 phosphorylates MiT/TFE proteins at key residues that creates a binding site for the 14-3-3, which sequesters the transcription factors inactive in the cytosol. Under nutrient deprivation conditions, inactive RagGTPases lead to mTORC1 inactivation and MiT/TFE protein dissociation from 14-3-3 as a consequence of their dephosphorylation mediated by the calcium-dependent activation of calcineurin. Nuclear accumulation of MiT/TFE proteins mediates the activation of a transcriptional network that promotes autophagy, lysosomal biogenesis, and increased lysosomal degradation. Upon nutrient replenishment conditions, MiT/TFE proteins nuclear export is regulated by mTOR-dependent phosphorylation and binding to CRM1/Exportin-1. The question marks denote that there is no direct evidence available to support the indicated processes for some of the MiT/TFE family members. CRM1, chromosomal maintenance 1; NPC, nuclear pore complex; P, phosphorylation; Rheb, Ras homolog enriched in brain; v-ATPase, vacuolar-type H+-ATPase.