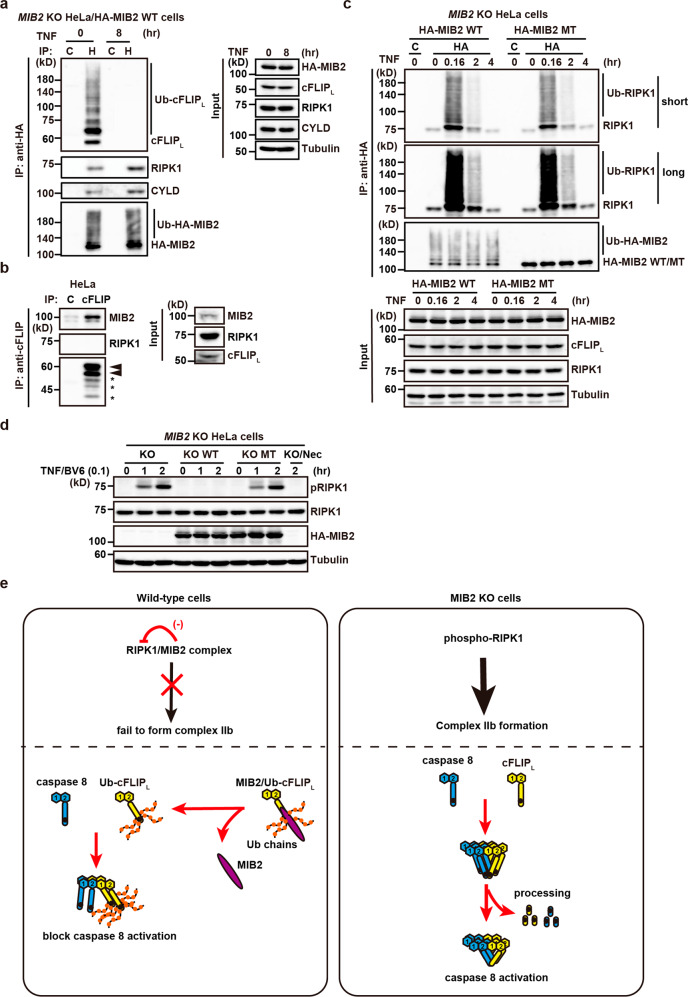
Fig. 10. Ubiquitin ligase activity of MIB2 is dispensable for RIPK1 ubiquitylation, but indispensable for suppression of RIPK1 kinase activity.
a MIB2 KO HeLa/HA-MIB2 WT cells were untreated or treated with TNF (10 ng/ml) for the indicated times, and then lysates were immunoprecipitated with control (C) or anti-HA antibody (H). Co-immunoprecipitated proteins were analysed by immunoblotting with the indicated antibodies. Protein expression was verified by the indicated antibodies using cell lysates. b The RIPK1/MIB2 complex and cFLIPL/MIB2 complex independently exist in the cells. HeLa cells were immunoprecipitated with control Ig (C) or anti-cFLIP antibody, and co-immunoprecipitated proteins were analysed by immunoblotting with the indicated antibody. The upper and lower arrowheads indicate modified and unmodified cFLIPL, respectively. Asterisks indicate degraded bands of cFLIPL. Protein expression was verified by the indicated antibodies using cell lysates. c MIB2 KO HeLa/HA-MIB2 WT or HA-MIB2 MT cells were untreated or treated with TNF (10 ng/ml) for the indicated times, and lysates were immunoprecipitated and analysed as in a. d MIB2 KO (KO), MIB2 KO HeLa/HA-MIB2 WT (KO WT), or HA-MIB2 MT (KO MT) cells were untreated or treated with TNF (10 ng/ml)/BV6 (0.1 μM) in the absence or presence of Nec-1s (20 μM) for the indicated times. Cell lysates were analysed by immunoblotting with the indicated antibodies. All results are representative of two or three independent experiments. e When WT cells are stimulated with TNF/IAP inhibitor, autophosphorylation of RIPK1 is prevented by MIB2, therefore RIPK1 cannot undergo complex IIb formation. Although caspase 8 and ubiquitylated cFLIPL are recruited to the RIPK1/FADD complex, ubiquitylated cFLIPL prevents a proper oligomer formation with caspase 8, thereby suppressing caspase 8 activation and apoptosis. In contrast, when MIB2 KO cells are stimulated with TNF/IAP inhibitor, autophosphorylation of RIPK1 is induced and phosphorylated RIPK1 subsequently forms the complex IIb. Recruited un-ubiquitylated cFLIPL forms a heterodimer with caspase 8 but fails to block caspase 8 activation, thereby inducing apoptosis.