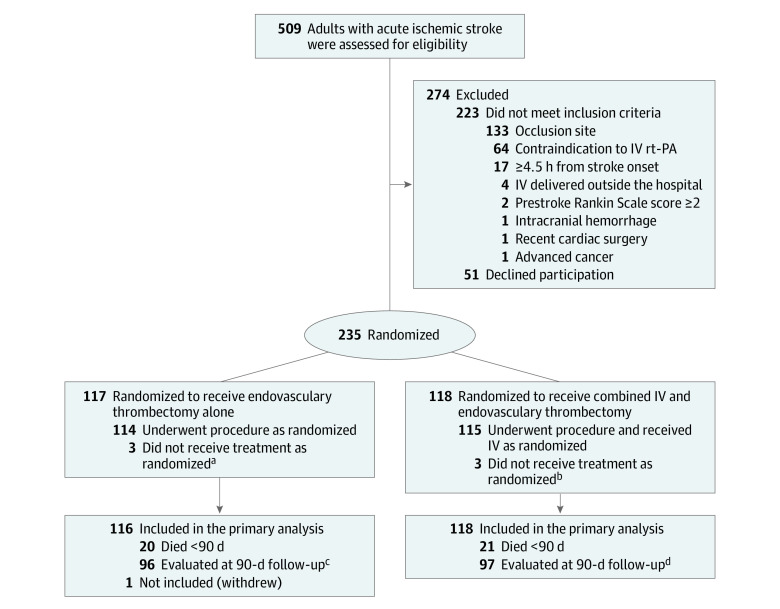
Figure 1. Eligibility, Randomization, and Follow-up of Patients Through the DEVT Randomized Clinical Trial of Intravenous Alteplase in Patients With Acute Ischemic Stroke.
aOne patient had expanded Thrombolysis in Cerebral Infarction (eTICI) 3 on the first intracranial angiography; the other, eTICI 2c on first intracranial angiography. One patient withdrew immediately after randomization.
bOne patient had eTICI 2c on first intracranial angiography; 2 had eTICI 2b on first intracranial angiography.
cOf the survivors, 91 had video recording, 4 had voice recording, 1 outcome determined by local investigators blinded to the treatment assignments because video or voice recording was unavailable.
dOf the survivors, 95 had video recording, 2 had voice recording, 0 outcomes determined by local investigators blinded to the treatment assignments.
DEVT indicates Direct Endovascular Thrombectomy vs Combined IV Thrombolysis and Endovascular Thrombectomy for Patients With Acute Large Vessel Occlusion in the Anterior Circulation Trial; IV indicates intravenous; rt-PA, recombinant tissue type plasminogen activator.