Figure 1. Participant Disposition.
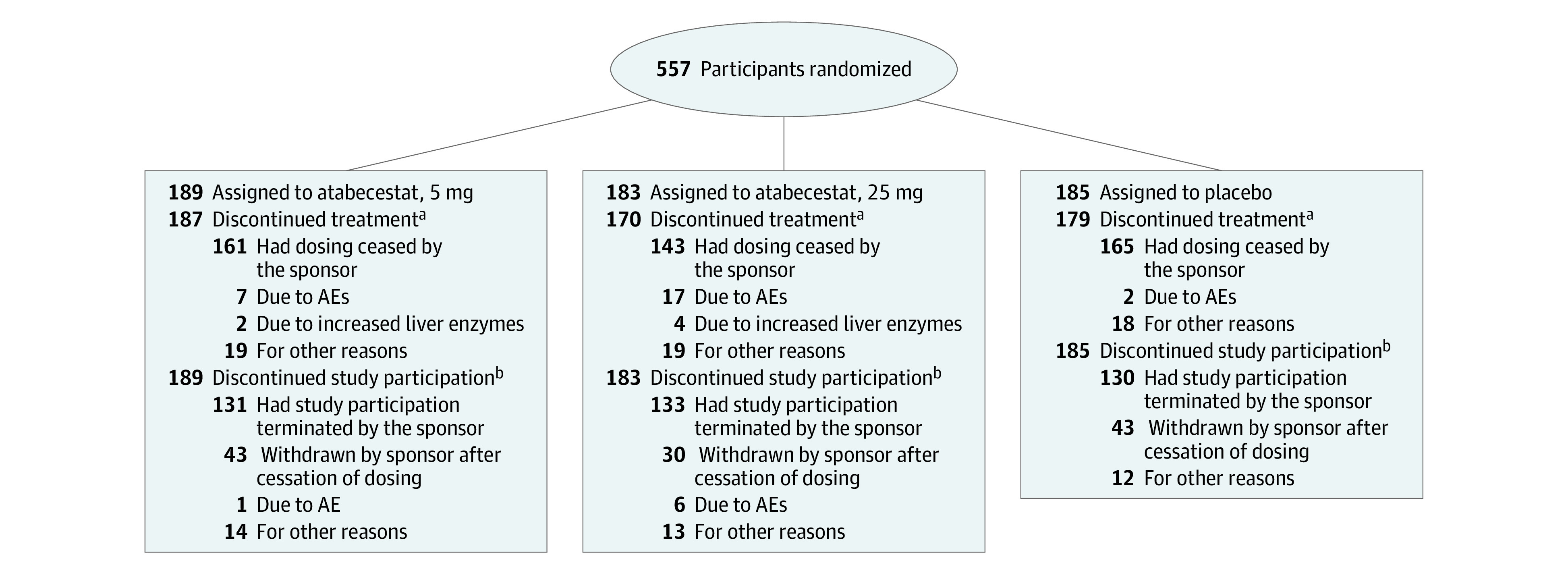
AE indicates adverse event.
aParticipants who discontinued study medication for other reasons before sponsor stopped atabecestat dosing.
bParticipants who discontinued the study. Participants could remain in the study after stopping study medication. Most study discontinuations were due to the sponsor stopping the study.
