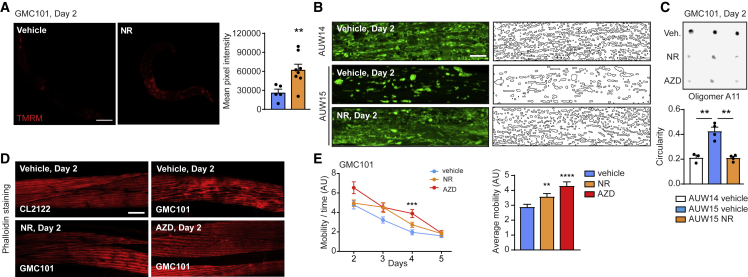
Figure 5.
NAD+ boosting after the induction of proteotoxic stress in muscle of Aβ-expressing worms reduces amyloid deposits and improves mitochondria and healthspan
(A) Confocal images of TMRM staining of aged (day 11) N2 worms treated ThT (50 μM; vehicle, n = 5; NR, n = 8 worms) and relative quantification. Scale bar, 100 μm.
(B) Representative confocal images of mitochondrial networks at day 2 and corresponding morphology analyses, including mitochondria outline and circularity assessment (in which 1 represents a perfect circle and 0 a line), in control AUW14 and Aβ1–42-expressing AUW15 worms treated with NR (1 mM) and AZD (300 nM; n = 20 per group). Scale bar, 10 μm.
(C) A11 dot blotting of day 2 GMC101 worms treated as indicated in Figure S4A, with NR (1 mM) and AZD (300 nM; n = 3 biological replicates per group). Relative quantification of the blot intensities is reported in Table S4.
(D) Confocal images of phalloidin-stained muscle fibers on day 2 in CL2122 (control) and GMC101 worms treated as indicated in Figure S4A (n = 8 worms per group). Scale bar, 10 μm.
(E) Spontaneous mobility and average mobility of GMC101 treated with vehicle, NR (1 mM), or AZD (300 nM) as in Figure S4A (vehicle, n = 38; NR, n = 37 worms; AZD, n = 36 worms). Overall differences between conditions were assessed by two-way ANOVA (average mobility); differences between conditions at individual time points were assessed by post hoc Sidak’s multiple comparison test.
See STAR methods for further details. Values in the figure are mean ± SEM. ∗p < 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001. Differences for two groups were assessed using two-tailed t tests (95% confidence interval) in (A), (B) (individual time points), (F), and (G). All experiments were performed independently at least twice. For all the individual p values, see the Table S6 (Excel data source Figure 5).