Figure 1.
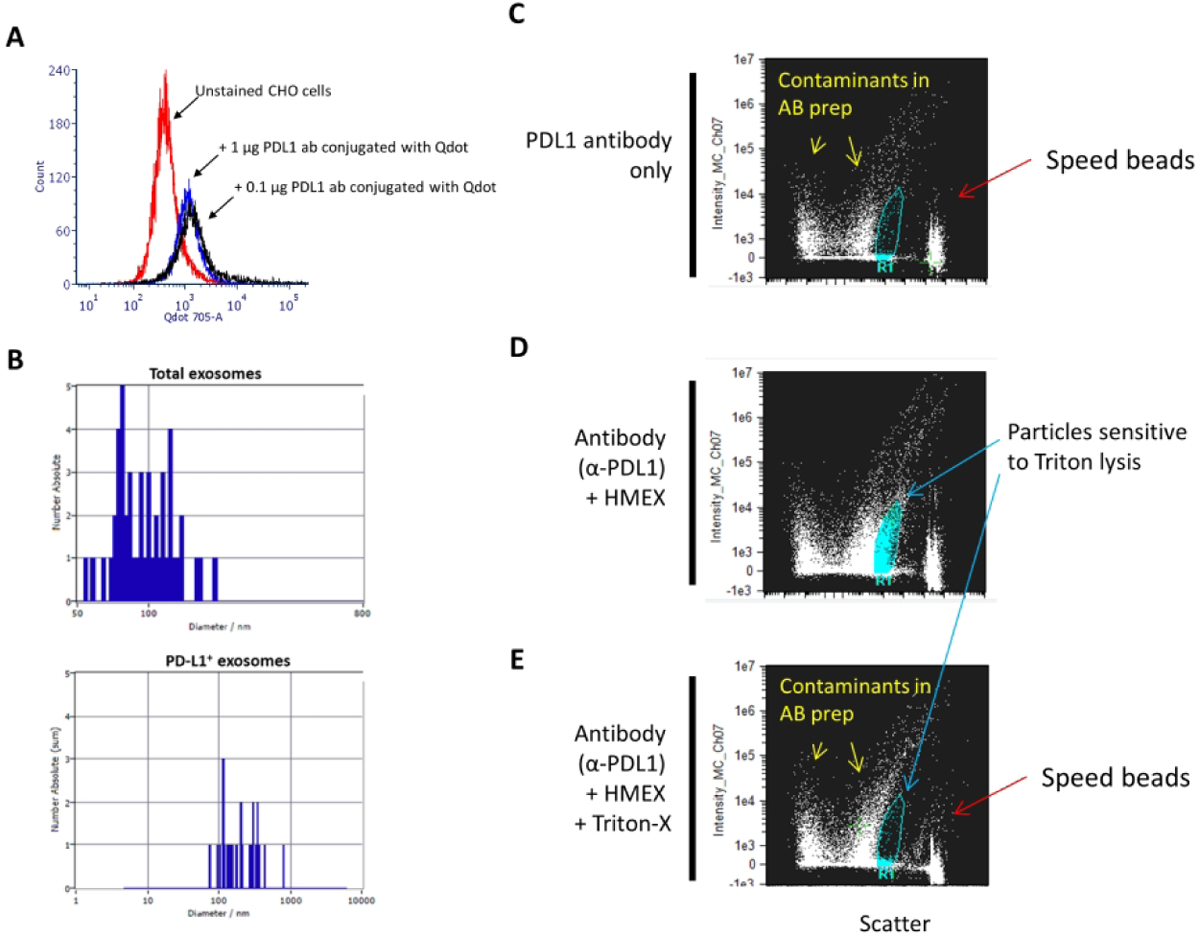
Detection of PD-L1 on HMEX A) Confirmation of successful conjugation of PD-L1 to Qdot 705 using SiteClick and post-purification process. Flow cytometry histogram of untreated PD-L1(+) CHO cells were used to set baseline for background/non-fluorescent signal, with positive signal determined by incubating 0.1 μg or 1 μg of conjugated PD-L1 Qdot 705 antibody with PD-L1(+) CHO cells. There is no observable difference in mean fluorescent intensity (MFI) between the two concentrations, indicating that 0.1 μg of antibody is a sufficient saturating concentration for detection of PD-L1 positive cells. B) Detection of a subpopulation of exosomes using fluorescent NTA. Total exosome number was measured using NTA. This was followed by fluorescent NTA with 688 +/− 20 nm bypass filter for detection of Qdot 705 conjugated PD-L1 antibody incubated exosomes. C–E) PD-L1 positive exosomes are membrane-bound vesicles that can be detected by ImageStream. This population is not detected upon treatment with a strong detergent (Triton-X). C) At the highest sensitivity settings, speed beads (~ 1 μm in size) are detected at the rightmost axis with a low scatter particulate banding pattern that is present when antibody, in absence of exosomes, is examined, indicating particulate contaminant patterns due to presence of antibodies. D) Detection of positive signal with intermediate scatter intensity indicative of PD-L1 positive exosomes. A distinct clustering of particulates is detected and present when PD-L1 antibody was co-incubated with exosomes. E) Loss of PD-L1 positive cluster of particulates upon addition of strong detergent (Triton-X), indicating that the clustered particles are membranous vesicles.
