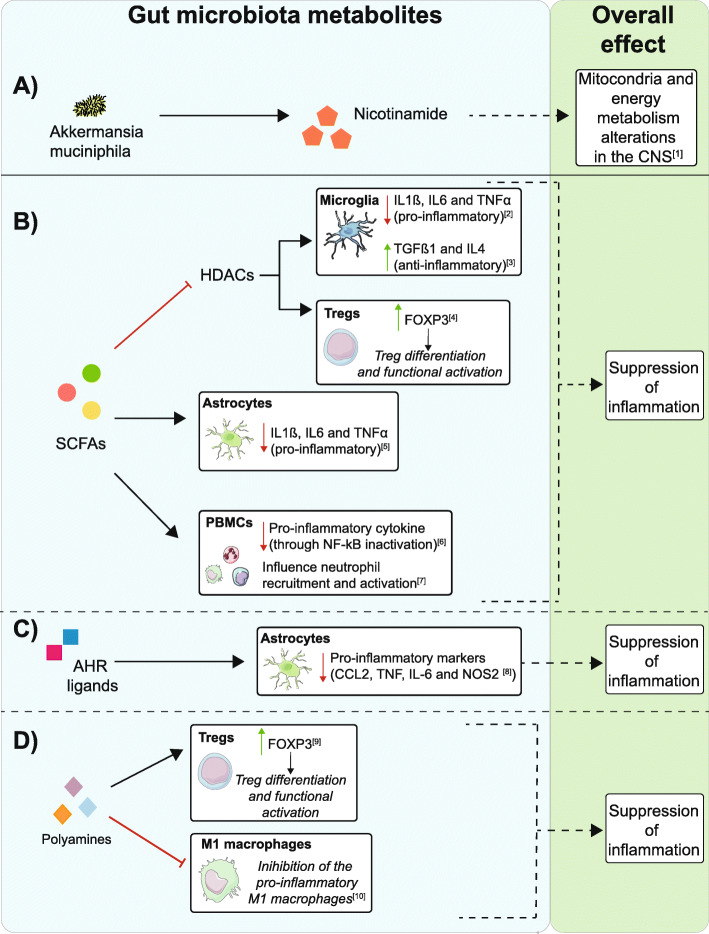
Fig. 2.
Metabolites produced by microbes found within the gut can influence neuronal health either directly or indirectly via CNS inflammation. a Metabolites released by the gut microbiome can enter the system circulation where they can access the CNS; in the case of nicotinamide released by Akkermansia muciniphila, this potentially modifies energy homeostasis and oxidative stress [17]. b–d A number of proposed mechanisms exist by which metabolites produced by microbes found within the gut can influence the immune response and have an effect of the CNS inflammatory state. b Short-chain fatty acids (SCFAs) can reduce inflammation by inhibiting HDACs within microglial cells, leading to the downregulation of pro-inflammatory (IL1ß, IL6 and TNFα) and upregulation of anti-inflammatory markers (TGFβ and IL4) [56, 57]. SCFA-mediated HDAC inhibition can also impact Tregs increasing their activity via upregulation of FOXP3 [58, 59]. SCFAs also influence astrocytes, reducing their inflammatory impact through downregulation of IL1ß, IL6 and TNFα [60]. Lastly, SCFAs exert anti-inflammatory effects on different peripheral blood mononuclear cells: they inhibit NF-kB leading to reduced pro-inflammatory cytokine production and immune cell recruitment and activation [61–63]. c Aryl hydrocarbon receptor (AHR) ligands can modulate astrocyte activities and give rise to anti-inflammatory properties [64]. d Polyamines induce FOXP3 expression in Treg cells promoting their differentiation and activation [65]. These molecules can also inhibit inflammatory macrophages (M1) thereby preventing macrophage-induced inflammation [66]