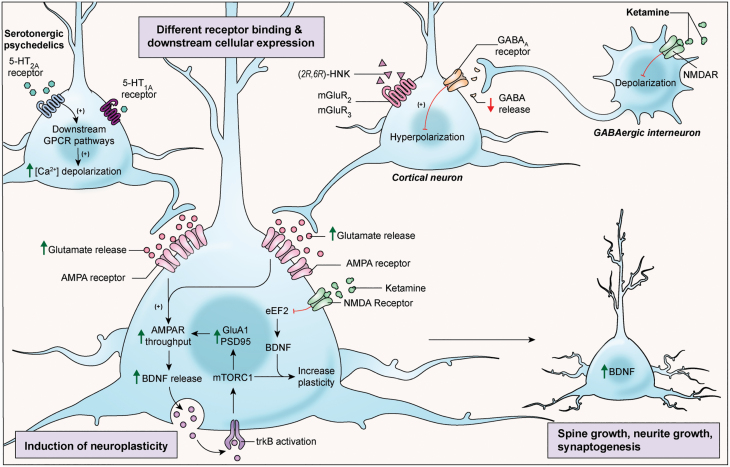
Figure 1.
The cellular mechanisms underlying ketamine and serotonergic psychedelics (SPs) may converge at glutamatergic synapses. The mechanism of action of SPs primarily begins with 5-hydroxytryptamine receptor 2A (5-HT2A) receptor agonism and activation (although 5-hydroxytryptamine receptor 1A [5-HT1A] agonism is also present) that, in turn, leads to glutamate release and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) potentiation, with subsequent stimulation of tropomyosin-related kinase B (TrkB), the primary target of brain-derived neurotrophic factor (BDNF), and an upstream activator of mechanistic target of rapamycin complex 1 (mTORC1). In contrast, ketamine selectively blocks a subset of N-methyl-D-aspartate receptors (NMDARs) localized to gamma aminobutyric acid (GABA)ergic interneurons, though the mechanisms underlying this action are not fully elucidated. This causes disinhibition of their glutamatergic (pyramidal) target neurons, triggering a surge in extracellular glutamate and correspondingly elevated non-cell autonomous glutamatergic synaptic transmission coupled with AMPA potentiation. Ketamine may also induce rapid BDNF translation in brain areas, in part through reduced phosphorylation and activation of eukaryotic elongation factor (eEF2). Growing evidence suggests that the (2R,6R)-hydroxynorketamine [(2R,6R)-HNK] metabolite acts independently of ketamine at presynaptic sites to promote glutamate signaling. As with SPs, the enhanced neural activity triggers BDNF release and is followed by transient activation of the mTOR pathway and increased expression of diverse proteins acting at glutamatergic synapses, along with functional strengthening of glutamatergic synapses. GPCR, G protein-coupled receptor; mGluR, metabotropic glutamate receptor; PSD95, post-synaptic density protein 95.