Fig. 1 |. Hit-to-lead optimization, pharmacological characterization and structure of allosteric modulator AS408 bound to β2AR.
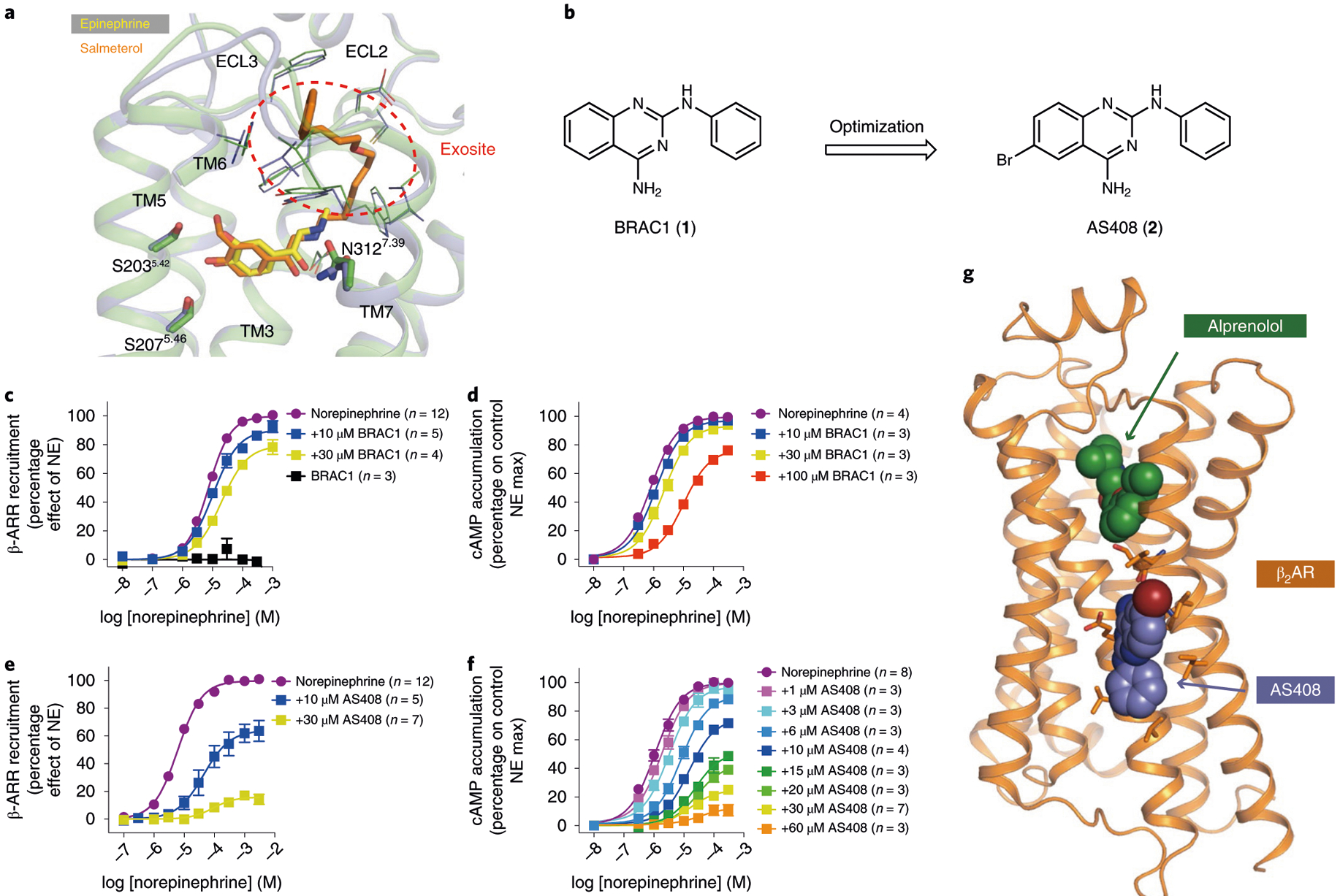
a, Salmeterol (orange) extends from the orthosteric site where epinephrine (yellow) binds to an exosite at the extracellular vestibule. The exosite was used as a docking template. b, Hit-to-lead optimization of the docking hit BRAC1. c–f, Negative allosteric effect of BRAC1 (c,d) and AS408 (e,f) on norepinephrine-stimulated β-arrestin 2 (β-ARR) recruitment (c,e) and on cAMP accumulation (d,f). Data are derived from 3–12 experiments done in duplicate and given as mean ± s.e.m.; the sample size (n) is labeled in the figure. g, Structure of AS408 bound to β2AR in the presence of antagonist alprenolol.
