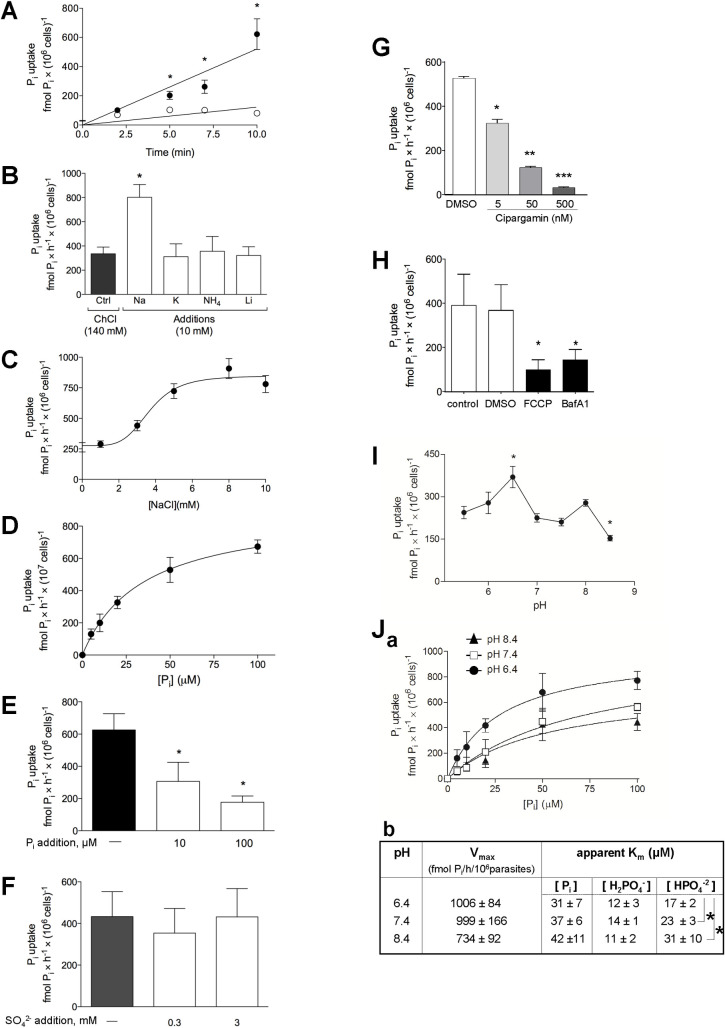
Fig 1. Characteristics of the transport of Pi in Toxoplasma.
A. Kinetics of Pi uptake. Extracellular parasites were incubated in a Pi-depleted reaction medium at pH 7.4 containing 100 μM 32Pi at the indicated times, before washing by filtration and radioactivity counting. Open circles: Na+-independent uptake in the presence of 140 mM choline chloride. Filled circles: Na+-dependent uptake in the presence of 140 mM NaCl. Data are means ± SEM of 4 independent assays. *, p<0.05 (unpaired Student’s t-test at matched time of reaction). B. Ion co-transport activities. 32Pi uptake was assayed on parasites incubated in a Pi-depleted medium containing radioactive Pi as indicated in A, in the presence of NaCl, KCl, NH4Cl or LiCl compared to choline chloride (control conditions). Data are means ± SEM (n = 3 independent assays). *, p<0.05 (unpaired Student’s t-test). C. Na+ dependence for Pi uptake. 32Pi uptake was assayed on parasites incubated in a Pi-depleted medium containing radioactive Pi as indicated in A, with increasing NaCl concentrations. Medium osmolarity was maintained at 300 mOsM with choline chloride supplementation. Data are means ± SEM (n = 6 independent assays). D. Saturation curve of Pi transport. 32Pi uptake by the parasites was monitored in medium with 140 mM NaCl and various concentrations of Pi at pH 7.4, and traced with 100 μM 32Pi (25 μCi/ml assay) for 30 min. Data are means ± SEM (n = 6 independent assays). E. Competition assay for Pi transport. 32Pi uptake by the parasite was monitored in medium with 140 mM NaCl and excess non-radioactive Pi at pH 7.4, and traced with 32Pi as described in D. Data are means ± SEM (n = 3 independent assays).*, p<0.05 (unpaired Student’s t-test). F. Selectivity for Pi influx. Extracellular parasites were incubated in a Pi-depleted reaction medium at pH 7.4 containing 100 μM Pi, traced with 25 μCi of 32Pi/ml for 30 min, with added of 0.3, 3 mM SO42- or no addition (−). Data are means ± SEM (n = 4 independent assays). G. Pi uptake upon inhibition of Na+-H+-ATPase. Extracellular parasites were treated 10 min with 5 nM, 50 nM, 500 nM cipargamin or without drug (DMS0 control) prior to Pi uptake, washed and incubated 10 min in the presence of 100 μM Pi and traced with 250 μCi of 32Pi at pH 7.4, in medium containing 140 mM NaCl. Data are means ± SEM (n = 3 independent assays). *, p = 0.0013; **, p = 0.0008; ***, p<0.0001 comparing with DMSO condition (unpaired Student’s t-test). H. Pi uptake upon condition of described in the presence of 100 μM Pi and traced with 250 μCi of 32Pi at pH 7.4, in a medium containing 140 mM NaCl, plus 10 μM FCCP, 100 nM bafilomycin A1, without drug (control) or vehicle (DMSO) for 10 min. Data are means ± SEM (n = 6 independent assays). *, p<0.05 comparing with DMSO (unpaired Student’s t-test). I. 32Pi uptake was measured on parasites in a Pi-depleted medium containing 140 mM NaCl and traced with 100 μM 32Pi (25 μCi/ml assay) for 30 min in different pH ranges. pH in the incubation medium was adjusted by adding concentrated HCl (5.5, 6.0 and 6.5) or KOH (7.0, 7.5, 8.0 and 8.5). The ratio H2PO4−/HPO42− varied from 50:1 at pH 5.5 to 1:20 at pH 8.5. Data are means ± SEM (n = 4 independent assays). *, p<0.05 (One-way ANOVA using the Tukey’s test, comparing each value with that obtained at pH 5.5). J. Panel a: pH-dependence of the influx of 32Pi into extracellular parasites resuspended in medium at pH 6.4, 7.4 or 8.4. Panel b: kinetic constants at pH 6.4, 7.4 or 8.4. The Vmax and Km values were extrapolated from the 3 curves in panel a. To determine the H2PO4- and HPO42- concentration dependences, the respective concentration in the medium of these ionic forms was calculated at the Pi concentrations shown in panel a, for each pH value: 6.4, 7.4 and 8.4. Asterisks, values obtained at the three different pH values differing significantly from one another (p<0.0001; One-way ANOVA using the Tukey’s test).