Proboscis extension–mediated waste clearance during deep sleep in Drosophila reveals a conserved primordial function of sleep.
Abstract
Sleep is a highly conserved state, suggesting that sleep’s benefits outweigh the increased vulnerability it brings. Yet, little is known about how sleep fulfills its functions. Here, we used video tracking in tethered flies to identify a discrete deep sleep stage in Drosophila, termed proboscis extension sleep, that is defined by repeated stereotyped proboscis extensions and retractions. Proboscis extension sleep is accompanied by highly elevated arousal thresholds and decreased brain activity, indicative of a deep sleep state. Preventing proboscis extensions increases injury-related mortality and reduces waste clearance. Sleep deprivation reduces waste clearance and during subsequent rebound sleep, sleep, proboscis extensions, and waste clearance are increased. Together, these results provide evidence of a discrete deep sleep stage that is linked to a specific function and suggest that waste clearance is a core and ancient function of deep sleep.
INTRODUCTION
Across the animal kingdom, sleep deprivation impairs learning, memory (1), and immune function (2) and delays wound healing (3), but a good night’s sleep can reverse these impairments. One of the great mysteries of sleep is how it fulfills these restorative functions and to what extent these mechanisms are conserved among the wide range of animals in which sleep has been identified and analyzed. We propose that those functions that are conserved represent the primordial functions of sleep that drove the evolution of this enigmatic state. One of these proposed functions involves waste clearance from the brain via sleep-triggered changes in fluid dynamics. In one model, cerebrospinal fluid (CSF) enters the brain parenchyma via periarterial pathways, driving convection of interstitial fluid (ISF) that removes toxic waste products from the brain’s interstitial space and drains along perivenous paths (4, 5). In addition, cerebral blood flow and blood volume decrease during slow-wave sleep (SWS), causing a temporary reversal in CSF flow direction in the third and fourth ventricles (6). These SWS-driven hemodynamic oscillations potentially facilitate waste clearance by allowing CSF and ISF to mix (6). Given the neuroanatomical specializations of mammalian brains, it was not clear whether this waste clearance function of sleep was also a feature of more divergent animals, including invertebrates.
While sleep has often been characterized in mammals by using neural signatures for different sleep stages, behavioral criteria are typically applied to a wide range of organisms. These behavioral criteria include behavioral quiescence that is reversible upon stimulation, a characteristic posture, decreased sensory responsiveness, and rebound sleep following sleep deprivation (7). Using these criteria, sleep has been found in nearly every animal that has been closely examined including invertebrates such as mollusks, cuttlefish, octopuses, crayfish, several species of insects, and even in a jellyfish, which lacks a centralized brain (8). Given the wide differences in brain structures and organismal behaviors, it is not clear whether sleep serves a common set of functions that would suggest ancient evolutionary origins. Moreover, if sleep serves similar functions, then it is far from clear how this might be accomplished given the wide diversity of brain structures. To address these questions, we have been studying sleep in an invertebrate model, the fruitfly Drosophila melanogaster.
Sleep in invertebrates has most of the hallmarks of mammalian sleep, including circadian and homeostatic regulation, where lost sleep is partially regained the next day (9, 10), increased arousal thresholds (9), a characteristic posture (10), and altered brain activity (11). Sleep in Drosophila is governed by similar neurotransmitters as in mammals, and flies respond to sleep- and wake-promoting drugs, including Gaboxadol [4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP)] (12) and caffeine (9), in a similar manner. Drosophila sleep has been shown to be important for learning and memory (12), energy conservation (13), reducing wake-induced performance degradation (14), and supporting immune functions (15). There is increasing evidence that invertebrate sleep also consists of different stages. Deep sleep in invertebrates has distinct neural correlates. Slow oscillations (~8 Hz), accompanied by a specific posture and increased arousal thresholds, were identified in crayfish (16), and 1-Hz oscillations in response to increased sleep pressure were observed locally in Drosophila R5 neurons (17). Bees that fall asleep progress through different postures that correlate with increased arousal thresholds (18) and fruitflies cycle through stages of lighter and deeper sleep within a sleep bout, as indicated by changes in arousal thresholds (19). Sleep initiation is a discrete brain process in Drosophila that is characterized by 7- to 10-Hz oscillations as the fly transitions into sleep (20). These observations suggest that partitioning sleep into discrete stages with specific functions is conserved in invertebrates as well. Yet, the functions of these different sleep stages and to what extent they are conserved between vertebrates and invertebrates remain obscure.
Here, we provide evidence of a deep sleep stage in Drosophila with a functional role in waste clearance. We find that, during sleep, flies occasionally enter a sleep stage that is characterized by stereotypical movement where flies repeatedly extend and retract their proboscis in the absence of gustatory stimuli. This is a deep sleep stage, as indicated by increased arousal thresholds and characteristic changes in neural activity. Preventing these proboscis extensions (PEs) increases mortality after injury and slows clearance of ingested or injected compounds. We propose that waste clearance is an ancient restorative function of deep sleep, where both flies and humans (6) have evolved mechanical solutions to increase hemodynamic oscillations during sleep.
RESULTS
PEs occur during a deep sleep stage
As part of studies examining electrical signatures of sleep (19), we noted occasional periodic movements of the proboscis. The proboscis is Drosophila’s feeding organ and is used for taste and food ingestion (21). Proboscis movements occur spontaneously in freely moving flies (movie S1) and have been observed using computer vision approaches (22, 23). This behavior appears to be different from the well-characterized Proboscis Extension Reflex (PER) (21), where stimulation of taste receptors on the fly’s legs initiates a prolonged extension of the proboscis. Unlike PER, flies are usually immobile and not in contact with food when the proboscis is repeatedly extended and retracted (movie S2). To study spontaneously occurring PEs, we tethered flies and placed them on an air-supported ball (movie S3), using an infrared camera to monitor fly activity (fig. S1A). Fly activity and PE were monitored using a pixel subtraction approach [c.f. (19)] in two regions of interest to determine fly movement (fig. S1A, blue box) and proboscis movement (fig. S1A, red box). PEs are defined as an extension of the proboscis, immediately followed by a retraction (Fig. 1A). This process takes ~1.4 s and resembles a fixed action pattern, where the amplitude is variable but the timing is constant (fig. S2). These proboscis movements differ from the PER where, upon detection of a gustatory stimulus, the proboscis is extended in a series of well-characterized steps (24). Upon PER initiation, the rostrum is lifted, the haustellum is flipped down, the labella is extended and spread, and eventually, the proboscis is retracted (movie S2 and fig. S3, A and B). PEs during sleep only go through these steps partially (fig. S3B). After the rostrum is lifted, the haustellum is flipped down partially and considerably slower (636 ± 46 ms versus 71 ± 6 ms), immediately followed by retracting the proboscis, much faster than during PER (1.4 ± 0.07 s versus 3.2 ± 0.4 s; fig. S3C). They also differ from fluid ingestion, where the proboscis is placed on a liquid source, which is ingested through cibarial pumping, while the proboscis remains extended (movie S2).
Fig. 1. PEs occur during a deep sleep stage.
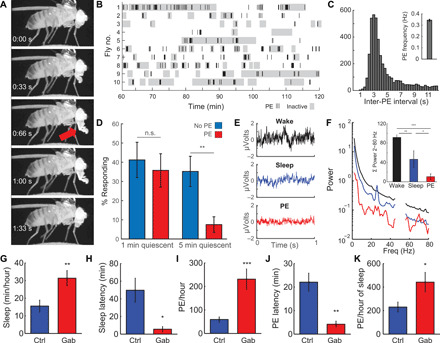
(A) A PE consists of a full extension of the proboscis (red arrow), immediately followed by a full retraction. This process takes ~1.4 s. (B) Raster plot showing PE (each bar, 1 PE) and inactivity (gray blocks, 60-s inactivity threshold) for 1 hour (61 to 120 min after being tethered). Most inactivity bouts contain one or more PEs. (C) During PE bursts, inter-PE intervals are highly regular, with one PE occurring every 3 s (inset; average PE frequency is 0.34 Hz). (D) Arousal thresholds for flies that were inactive or inactive and making PE. After 5, but not 1, min of inactivity, flies making PE have greatly increased arousal thresholds. n = 8 to 13 per group; **P < 0.01, t test. n.s., not significant. (E) Representative 1-s traces for LFPs during wake, sleep, and PE. (F) Average power spectra of LFPs for flies that were awake, asleep, or producing PE show that LFP power is lower during PE compared to wake or sleep (inset). The data have been notch-filtered at 50 Hz (Australian line noise). n = 9; *P < 0.05, **P < 0.01, and ***P < 0.001 [analysis of variance (ANOVA) with Bonferroni test]. (G) Feeding flies Gaboxadol food (0.2 mg/ml) induces sleep (H) and decreases sleep latency (occurrence of first sleep bout after being tethered). (I) PEs per hour are greatly increased. (J) PE latency, the time to detection of the first PE after being tethered, is decreased. (K) Amount of PE per hour of sleep is increased after Gaboxadol administration. n = 15 control and n = 9 Gaboxadol; *P < 0.05, **P < 0.01, ***P < 0.001, two-tailed t test. Error bars indicate SEM. All error bars indicate SEM.
We found that flies usually are inactive during PE (Fig. 1B), where inactivity refers to the lack of leg, wing, or grooming motions. Using the classic 5-min (300 s) inactivity criterion for Drosophila sleep, 94% of these sleep bouts (136 bouts in 25 flies) contain PEs in our tethered paradigm. PEs can either occur as single isolated events or as prolonged bursts of up to 15 or more PEs (fig. S1, B and C). During these bursts, a PE occurs about every 3 s (Fig. 1C), with an average frequency of 0.34 Hz (Fig. 1C, inset). Approximately 52% of proboscis extensions (PEs) occur as single events, while the remainder occurs as bursts of two or more consecutive PEs (fig. S1C). The longer the burst duration, the more likely a fly is to be inactive (fig. S1D).
Although our video observations (movie S1) suggest that flies frequently exhibit PE during extended periods of inactivity, does this also mean that the fly is asleep? Sleep in Drosophila is characterized by immobility, increased arousal thresholds, altered brain activity, and homeostatic regulation. We next determined whether these flies exhibit an elevated arousal threshold consistent with sleep. To test whether arousal thresholds are increased during PE, we [c.f. (19)] delivered vibratory stimuli (200 ms, 1.2 g) to inactive tethered flies that were making PEs. After 1 min of inactivity, response rates for both groups are ~40% (Fig. 1D). There is no change in responsiveness between flies that have been inactive for 1 or 5 min. However, after 5 min of inactivity, it becomes almost impossible to rouse flies soon after making PEs, as their response rate drops below 10% (Fig. 1D), much lower than control flies not engaged in PE. These results demonstrate that arousal thresholds to mechanical stimuli are greatly increased during PE sleep (PES). Since we did not test arousal thresholds to other sensory modalities, we cannot exclude the possibility that these may have a different impact. Nonetheless, these results demonstrate that arousal thresholds to mechanical stimuli are greatly increased and that sleep during PE is deeper, compared to flies that are inactive for a similar duration but not making PE.
Besides immobility and increased arousal thresholds, Drosophila sleep is characterized by reduced activity in the brain, as indicated by decreased power of local field potentials (LFPs) recorded in the fly brain (11), where deeper sleep correlates with reduced LFP power (19). If sleep during PEs is deeper, we hypothesized that LFP power during these stages is decreased. To test this, we reanalyzed a dataset consisting of fly LFP along with movies of fly behavior during these recordings (19). PEs were detectable in 10 of 13 flies (movie S4). We manually scanned through all video data of those 10 flies to detect epochs with PEs. LFP in these epochs was compared to LFP during wake or sleep (i.e., inactive for >5 min). LFP power during sleep was lower than during wake (Fig. 1, E and F). In addition, LFP power during periods of PE epochs was even lower than during regular sleep, further cementing the observation that PEs occur during a deep sleep stage (Fig. 1, E and F).
To validate whether PEs occur during deep sleep, we tested the effect of pharmacological deep sleep induction on PE. Gaboxadol, a γ-aminobutyric acid type A agonist, produces SWS in humans (25) and induces deep sleep in Drosophila that is accompanied by increased arousal thresholds (12, 26) and reduced LFP power (20). To test whether Gaboxadol administration affects PE, we fed flies food laced with Gaboxadol (0.2 mg/ml) and tested its effect on PE. As expected, Gaboxadol increases sleep (Fig. 1G). Increased sleep pressure is reflected in decreased sleep latency, the time it takes a fly to fall asleep after lights off (27). Here, Gaboxadol administration decreases sleep latency in tethered flies (Fig. 1H), indicating that Gaboxadol causes flies to fall asleep sooner. This is accompanied by an increase in PEs (Fig. 1I) and decreased PE latency (time after tethering until the first PE is detected; Fig. 1J). Increased PE after Gaboxadol administration is not just due to increased total sleep, as the amount of PE per hour of sleep increased as well (Fig. 1K). Thus, Gaboxadol not only causes flies to enter deep sleep sooner; it also supports the idea that PEs are part of a deep sleep stage. Given these observations, we hypothesize that PEs define a discrete stage of sleep.
PEs are under circadian and homeostatic control
Drosophila sleep is under circadian and homeostatic control, where, at the start of the night, flies sleep the most, presumably because of sleep pressure building up during the day. Then, during the night, sleep gradually decreases as sleep pressure dissipates. To test whether PEs follow a sleep-like pattern, we tethered flies every 3 hours and quantified sleep and PE. Both sleep and PE per hour are low at circadian time 0 (CT0) and CT9 and highest at CT12 (Fig. 2, A and B). PE per hour decreases steadily across the subjective night, possibly because of discharge of homeostatic drive by PE. This is also reflected in arousal threshold data indicating that sleep is deepest (most elevated arousal threshold) at the start of the night and then decreasing as sleep pressure dissipates (19). To test whether PEs are under homeostatic regulation, we sleep-deprived flies for 12 hours during the dark phase. Immediately after sleep deprivation, flies were tethered, and sleep and PE were quantified from Zeitgeber Time 1 (ZT1) to ZT3. Rebound sleep was observed in our tethered preparation, as sleep significantly increases and sleep latency is reduced, compared to nondeprived controls (Fig. 2, C and D, and fig. S4, A and B). Likewise, the number of PEs per hour increased greatly during the first 2 hours of rebound sleep (Fig. 2E). In addition, the latency to the first PE occurrence is reduced in the sleep-deprived group (Fig. 2F). Together, these results suggest that PEs are under homeostatic control. In conclusion, PEs seem to indicate the presence of a deep sleep stage that is under circadian and homeostatic control. During PE, flies are immobile, arousal thresholds are increased, and LFP is decreased. We term this stage as PES.
Fig. 2. PEs are under circadian and homeostatic control.
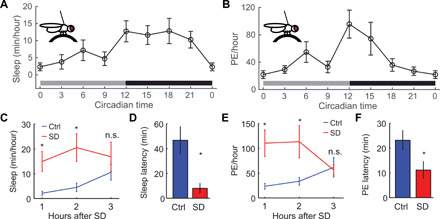
(A) Sleep in tethered flies in constant darkness follows a circadian pattern, where sleep is higher during the night than during the day (n = 15 flies per time point). (B) PEs in tethered flies increase during the day and are highest at the start of the subjective dark phase (CT12) and gradually dissipate throughout the night, although sleep remains high (n = 15 flies per time point). (C) After sleep deprivation (SD), sleep increases (*P < 0.05; ANOVA with Bonferroni test), (D) while sleep latency decreases (*P < 0.05, t test). (E) Likewise, the amount of PE is strongly increased for the first 2 hours of rebound sleep (*P < 0.05, n.s., ANOVA with Bonferroni test) (F), and the latency to detecting the first PE after being tethered decreases (*P < 0.05, t test). (C to F) n = 10 per group.
PEs facilitate recovery from injury
We observed that dying flies in our tethered preparation display much higher amounts of PE during the last 2 hours of their lives, compared to the first 2 hours of being tethered (fig. S5, A to C), raising the possibility that PEs are part of a physiological stress response. Likewise, scientists performing imaging experiments on the brains of live, tethered flies reported having to immobilize the proboscis, as PEs occur frequently enough to interfere with the recordings (28, 29), suggesting that PEs could be part of an injury-induced response caused by opening the head capsule. If PEs are part of an injury response, we hypothesized that inducing acute injury should increase PEs. To test this, we used the high-impact trauma (HIT) assay (30) to induce full-body injury in flies (Fig. 3A). After injury induction, a single, outwardly intact fly (i.e., no observable damage to the cuticle, legs, or wings) was selected and tethered. This type of acute, internal injury did not affect total sleep in tethered flies (Fig. 3B) but resulted in an immediate fivefold increase in PEs per hour (Fig. 3C).
Fig. 3. PEs facilitate recovery from injury.
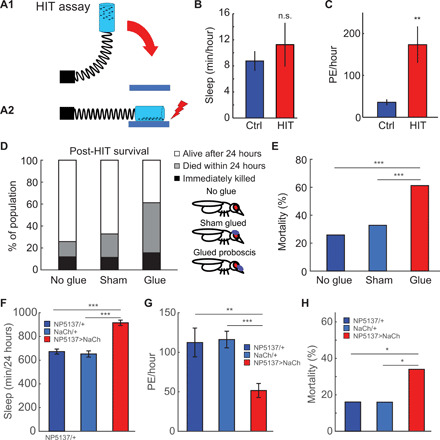
(A) Full-body injury was induced using the HIT assay (30), where flies are loaded in a vial attached to a spring. By pulling back the spring (A1) and releasing it, the vial slams on a rubber block, causing full-body impact injuries (A2). (B) Flies were immediately tethered after injury and sleep, and PEs were measured over 3 hours postinjury, showing no effect on sleep (n.s., t test) (C) but a strong increase in PEs per hour (**P < 0.01, t test; n = 9 per group). (D) To test whether PE affects survival rate, the proboscis was immobilized with UV-cured glue. Sham-treated flies received glue on top of the head, covering the ocelli but leaving the proboscis free. Preventing PE increases the proportion of flies that die within 24 hours after injury. The proportion of flies immediately killed does not differ between groups. (E) Immobilizing the proboscis increases 24-hour mortality (n = 119, n = 88, and n = 130; ***P < 0.001, chi-square test). (F) Constitutively activating neurons controlling feeding (NP5137-Gal4) using NaChBac increases sleep and (G) decreases PE, compared to parental controls (n = 50 per group; **P < 0.01 and ***P < 0.001, ANOVA with Bonferroni t test), (H) and increases mortality after injury (n = 50 per group; *P < 0.05, chi-square test). All error bars indicate SEM.
To test whether this increase in PEs confers some benefit, we immobilized the proboscis by placing light-cured glue on the rostrum and haustellum (the base and the middle parts of the proboscis) but leaving the labella free, allowing flies to feed. This completely prevents PEs/retractions from occurring (movie S5). In the sham-treated group, a similar amount of glue was placed directly on top of the head (Fig. 3D). After immobilizing the proboscis this way, flies were given 3 days to recover; this also eliminates all flies unable to feed. To test whether feeding is affected after this recovery period, we used a feeding-based excretion assay (31), where food consumption is measured by feeding flies food colored with Blue #1, a nontoxic food coloring dye that is not metabolized and not absorbed in the gut (31). Thus, excretion of the dye reflects feeding and excretory processes. Three days after immobilizing the proboscis, flies were placed on agar with 5% sucrose and 1% Blue #1 for 24 hours. Afterward, groups of flies were transferred to glass scintillation vials every hour until blue excretion was no longer observed. There was no difference in excretion per hour (fig. S6A) or cumulative excretion (fig. S6B), between proboscis-immobilized flies and sham-treated controls, indicating that immobilizing the proboscis does not impair overall food consumption or excretion of ingested substances.
To test whether preventing the PEs that normally occur after acute injury affects survival, we immobilized the proboscis as described, then induced full-body injury with the HIT assay. Completely immobilizing the proboscis greatly increases the number of flies that die within 24 hours after injury, compared to untreated or sham treated controls (Fig. 3, D and E). To test whether decreasing, rather than preventing, PE affects mortality, we used UAS-NaChBac (32) to constitutively depolarize NP5137-Gal4, which is expressed in a pair of command neurons initiating feeding behavior (33). This increases total sleep (Fig. 3F) and decreases PE (Fig. 3G) in NP5137>NaChBac, compared to parental controls. NP5137>NaChBac flies are less able to recover from full-body injury, as mortality is almost double that of parental controls (Fig. 3H). Together, these results demonstrate that PEs are part of an injury and/or physiological stress–induced response, where PEs increase markedly within minutes after injury, and decreasing or preventing PE increases mortality after injury.
PEs facilitate waste clearance
PEs could be adaptive and improve responses to stress such as injury by improving clearance of waste and other byproducts of stressful events, such as tissue injury. In Drosophila, waste clearance occurs through the Malpighian tubules (MTs), the fly homolog of the kidney [reviewed in (34)]. The MTs float in the hemolymph, collect waste products, and shunt them into the hindgut, where they are excreted. Repeated PEs can facilitate this process by increasing convective hemolymph bulk flow along the MTs (Fig. 4A), similar to how the glymphatic system is proposed to increase convective CSF bulk flow (35).
Fig. 4. PEs facilitate clearance from hemolymph.
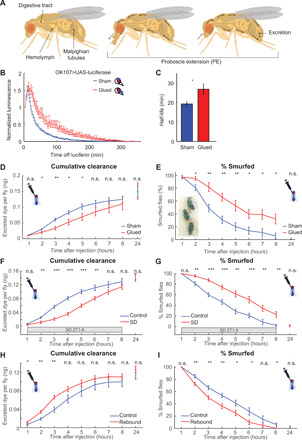
(A) Model of how PE facilitates waste clearance. As the proboscis is extended, hemolymph moves forward, reversing when the proboscis is retracted. PE drives convective hemolymph along the MTs, where waste is absorbed, shunted into the gut, and excreted. (B) After transfer to nonluciferin food, luminescence generated by OK107>luc flies gradually decreases. This is slower in glue-immobilized flies, compared to sham-treated controls (n = 36 to 48 per group, two replicates). (C) As indicated by increased luminescence half-life (*P < 0.05, t test). (D) Glue-immobilizing the proboscis reduces the rate at which injected dye is cleared. However, the total amount of excreted dye in 24 hours is not different (n = 8 to 12 per group, nine replicates). (E) Within seconds after being injected, flies smurfed (inset). Immobilizing the proboscis caused flies to remain smurfed longer. Twenty-four hours after injection, no smurfed flies were detected. (F) Sleep deprivation decreases the rate at which injected dye is cleared (n = 8 to 12 per group, eight replicates) and (G) causes flies to remain smurfed longer. (H) Sleep deprivation before injection increases the rate at which injected dye is cleared (n = 8 to 10 per group, eight replicates). (I) Rebound sleep causes flies to lose their smurfed status sooner. All error bars indicate SEM. (D to I) *P < 0.05, **P < 0.01, and ***P < 0.001, n.s., ANOVA followed by t tests.
To test whether PEs facilitate waste clearance, we first used a luciferase assay, where transgenic firefly luciferase converts exogenously provided luciferin into oxyluciferin and light (fig. S7A) (36). Luciferin is a small molecule that passes from the gut into the hemolymph and through the blood-brain barrier (BBB), as luciferase-induced luminescence can be detected in the fly nervous system within 10 to 20s after consuming luciferin food (37). Because luciferase activity is short-lived, we can use luciferase activity to infer how much luciferin is present in the fly in real time by imaging bioluminescence (fig. S7, B and C). UAS (upstream activation sequence) luciferase was expressed in OK107-Gal4, which expresses strongly in the mushroom body in the fly central brain (38). Flies were prefed with food laced with 5 mM luciferin for 24 hours, after which the proboscis was immobilized, and flies were transferred to a microplate containing regular agar-sucrose food, but no luciferin, and placed in a luminescence counter. In control flies, bioluminescence levels declined over time with a half-life of ~20 min presumably as luciferin gradually gets metabolized and excreted. Immobilizing the proboscis slowed this decay rate in luminescence (Fig. 4B), as reflected by an increased half-life (Fig. 4C).
As changes in decay rates could be caused by changes in luciferin metabolism rather than clearance, to more directly test whether immobilizing the proboscis affects excretion, we injected flies with 1% Blue #1, a nonmetabolizable dye (31). As we are injecting rather than feeding as in fig. S6, we are bypassing the gut barrier and thus can assess excretion independent of feeding. After injection, flies are immediately transferred to scintillation vials and then transferred hourly to fresh vials to collect excreted dye (fig. S7D). Afterward, the amount of excreted dye is quantified. Abolishing PEs slows excretion during the first 6 hours after injection (Fig. 4D). The total amount of dye excreted in 24 hours does not differ between proboscis-immobilized flies and sham-treated controls, indicating that all injected dye is eventually cleared (Fig. 4D). Injecting flies with 50-nl phosphate-buffered saline (PBS) containing 1% Blue #1 turns flies completely blue within seconds as the dye spreads through the hemolymph (Fig. 4E, inset). By visually inspecting flies every hour, we determined how many flies are “smurfed,” i.e., where the blue dye can be observed in the head, thorax, and abdomen. The number of smurfed flies reduced more slowly in the proboscis-immobilized group (Fig. 4E), further validating that PEs facilitate waste clearance.
Our preceding experiments suggest a link between PE during sleep and a role for PE in waste clearance. To test whether preventing sleep affects waste clearance, we injected flies with 50-nl Blue #1 and sleep-deprived them (from ZT1 to ZT8), while they were placed in scintillation vials to collect excreted dye. Although sleep loss cannot be directly quantified while flies are placed in vials, we measured rebound sleep after 8 hours of sleep deprivation in vials and compared this to flies that were sleep-deprived in Drosophila Activity Monitors, where sleep loss can be quantified. Sleep deprivation resulted in strong sleep loss in activity monitors (fig. S8A). Sleep deprivation in both activity monitors and vials resulted in robust rebound sleep from ZT9 to ZT12 (fig. S8, A and B), indicating that flies lost a comparable amount of sleep. Sleep deprivation reduces the rate at which injected dye is cleared (Fig. 4F), and sleep-deprived flies remained smurfed longer, suggesting that loss of sleep during sleep deprivation is accompanied by a loss of waste clearance function.
After sleep deprivation, flies show rebound sleep, consisting of increased sleep and PE, as well as lower latencies to both (Fig. 2, C to F). To test whether increased sleep and PE during the rebound phase are functionally related to waste clearance, we sleep-deprived flies overnight and injected them with 50-nl Blue #1 in PBS immediately afterward. During the rebound phase, clearance of the injected dye was increased, compared to non–sleep-deprived controls (Fig. 4H). Likewise, during rebound sleep, flies lost their smurfed status faster (Fig. 4I). Together, these results cement the relationship between sleep and PE-mediated waste clearance, a sleep function.
DISCUSSION
Our data suggest that repetitive proboscis extensions (PEs) and retractions during sleep indicate the presence of a previously unidentified, discrete deep sleep stage in Drosophila, termed Proboscis Extension Sleep. This stage is characterized by increased arousal thresholds, decreased LFP power, and circadian and homeostatic regulation. Preventing PEs slows clearance of injected dye and ingested luciferin from the hemolymph and increases mortality after full-body injury, indicating that Proboscis Extension Sleep has a functional role in waste clearance. The finding that a deep sleep stage serves a role in waste clearance in the fruitfly indicates that waste clearance is an evolutionarily conserved core function of sleep and suggests that waste clearance may have been a function of sleep in the common ancestor of flies and humans. Both flies and humans seem to have independently evolved mechanisms to maintain this function within the constraints of their anatomy and other features of sleep, e.g., slow waves may be specializations to sustain this function in vertebrate lineages.
How can proboscis movements facilitate waste clearance? Since the fly’s proboscis is quite large [~20% of the head’s volume; (39)], extending and retracting the proboscis will move a substantial amount of hemolymph through the body (Fig. 4A). Thus, PEs can increase hemodynamic oscillations in the fly, increasing hemolymph flow along organs and tissues, which is likely to facilitate hemolymph’s transport functions, nutrient delivery, and waste clearance (movie S6). Because PEs increase after sleep deprivation/spontaneous wakefulness, we hypothesized that the accumulation of wake-related metabolic waste products stimulates PEs during sleep. Consistent with this hypothesis, PEs also increase clearance of the metabolic waste product carbon dioxide in flying insects (40), by driving hemolymph back and forth creating internal pressure differences on the trachea system, adding an active component to Drosophila respiration that can be recruited when passive respiration—opening spiracles to allow gas exchange—is not sufficient. However, during sleep, the fly’s metabolic rate is lower than during wake (13), which makes it unlikely that dumping CO2 is the main function of PE during sleep.
Drosophila BBB permeability peaks at ZT12 (41), at the same time when sleep and PE peak (Fig. 2, A and B). In addition, sleep promotes endocytosis at the Drosophila BBB, where endocytosis is highest early on in the night (ZT14) and low at ZT18 (42), time points that coincide well with our observations on PE (highest at CT12 and low at CT18; Fig. 2B). Thus, PE-induced hemodynamic shifts may be coordinated with BBB permeability to facilitate waste clearance during sleep. In mice, chronic sleep restriction impairs BBB functions (43). In Drosophila, sleep deprivation delays the peak of BBB endocytosis, which normally occurs at the beginning of the night, to occur during rebound sleep instead (42), similar to what we observe in changes to PE after sleep deprivation (Fig. 2, C to F) and clearance of an injected dye from the hemolymph, which is reduced during sleep deprivation but increased during subsequent rebound sleep (Fig. 4, F to I).
Persistent depolarization of a pair of motor command neurons regulating feeding (33) decreases PE and, interestingly, increases total sleep (Fig. 3, F and G). These data suggest that sleep drive increases as a sleep function, waste clearance, is impaired. A similar effect is observed when flies are deprived of sleep overnight, resulting in increased sleep accompanied by increased PE (Fig. 2, C and E) during the rebound phase. We also demonstrated that waste clearance is reduced during sleep deprivation (Fig. 4, F and G), linking loss of sleep to a decrease of this sleep function. Thus, these increases in sleep, observed in both NP5137>NaChBac and sleep-deprived flies, are likely to be a compensatory response to the inability to carry out sleep’s restorative functions, possibly including waste clearance. This idea is further supported by the observation that waste clearance is increased during post–sleep deprivation rebound sleep (Fig. 4, H and I).
Acute injury also increases PE, perhaps via molecules released by cellular injury (e.g., damage-associated molecular patterns), stress response genes, cytokines, or other immune factors. Neural injury triggers several responses, including glial migration and phagocytotic clearance of damaged neurons. After injury, it is possible that PEs facilitate transport of hemocytes—Drosophila blood cells that engulf and melanize foreign materials and secrete antimicrobial peptides—and other immune factors to the injury site or clear maladaptive injury-released molecules. Recent work in Drosophila demonstrated that antennal ablation increases sleep, that sleep deprivation slows down clearance of damaged axons, and that sleep induction through Gaboxadol speeds up accelerated clearance of ablated neurons (44, 45), further cementing the link between injury responses and sleep.
These results demonstrate a key conserved process in the neurophysiology of sleep, where mammals and invertebrates evolved different mechanisms to increase macroscopic fluid flow during sleep. Neuroimaging in humans showed that during SWS, synchronized waves of neural activity decrease cerebral blood flow, causing a temporary drop in cerebral blood volume that allows retrograde CSF to flow into the third and fourth ventricles. These macroscopic CSF oscillations during SWS may contribute to the disposal of waste products, as pulsatile fluid dynamics can increase mixing and diffusion (6). While SWS per se has not been described in flies, ~1-Hz oscillations in membrane potential have been observed in R5 ellipsoid body neurons, important for sleep homeostasis (17). It will be of interest to determine whether R5 oscillations are functionally linked to PEs. Nonetheless, it is notable that hemodynamic oscillations in macroscopic fluid flow during deeper sleep states may be a primordial feature of sleep. The finding of waste clearance as a sleep function in both vertebrates and invertebrates suggests that it is an important driver of sleep evolution.
MATERIALS AND METHODS
Animals
Flies (D. melanogaster) were reared on standard fly medium and kept at 25°C under a 12-hour light/12-hour dark cycle. Adult female isogenized w1118 (3 to 7 days after eclosion) were used for all experiments. NP5137-GAL4 was obtained from Kyoto. Repo-Gal4 was obtained from Bloomington (w[1118]; P{w[+m*]=GAL4}repo/TM3, Sb[1] #7415). UAS-NaChBac was obtained from M. Nitabach. UAS-luciferase was obtained from N. Perrimon. OK107-Gal4 was obtained from A.-S. Chiang.
Fly tethering
Flies were tethered using a protocol similar to (46). Female flies were briefly anesthetized on ice and transferred to a cold plate and then glued to a wire hook at the thorax (made from Fine Science Tools, item no. 26002-10), using light-cured super glue [SuperGlue ultraviolet (UV) glass adhesive, 505127A, Pacer Technology, Cucamonga, CA, USA]. Glue was cured by 15-s application of low-intensity UV flashlight (Solarez, Vista, CA, USA). The tethering process takes less than 1 min. Typically, flies immediately start flying after waking up from cold anesthesia. Flies that did not show flying behavior or took more than 30 s to wake up were discarded. Flies are then placed on an air-suspended ball, using a micromanipulator consisting of the orthogonal linear stages, situated into a small incubator kept at 25°C. For circadian experiments, flies were tethered under red light at CT11 and CT17 to avoid the effects of light on sleep and circadian rhythms.
Quantifying fly activity
Flies are placed on a small air-suspended ball and filmed under infrared illumination (850 nm) at three frames per second using an infrared camera (Point Grey, Chameleon). All experiments were carried out in darkness, to avoid the arousal effects of light. Activity was quantified offline using a pixel subtraction approach [c.f. (27)] in two regions of interest to determine fly movement (fig. S1A, blue box) and proboscis movement (fig. S1A, red box).
Quantifying PEs
PEs were quantified offline, by manually scanning through the videos and using custom-made MATLAB (MathWorks) software. To identify PEs, we scrolled through the tethered fly videos, using VirtualDub (virtualdub.org) while simultaneously plotting the Δpixel trace for the proboscis region of interest (fig. S1A, blue box), 1000 frames at a time. PEs appear as large peaks in this trace (fig. S1B) and were marked by clicking on the highest amplitude in the Δpixel trace, denoting the timing and amplitude of each PE. Users were blinded to the experimental conditions.
Arousal testing in tethered flies
To test behavioral responsiveness during PEs, we monitored fly activity using a real-time tracking algorithm written in MATLAB to measure fly activity and proboscis movements. When the fly is making PEs while being inactive for 1 or 5 min, a 200-ms, 1.2-g vibration stimulus is generated by a 12-mm shaftless vibrating motor (Pico Vibe 312-101; Precision Microdrives) fixed to the tethering post.
Two-channel differential LFP
LFPs were recorded as described (19).
Pharmacological sleep induction
For sleep induction experiments, flies were placed overnight on 1% agar and 5% sucrose food laced with Gaboxadol (0.2 mg/ml; Sigma-Aldrich). A little blue food coloring was added to the food, so flies who had recently fed could be readily identified by their blue bellies.
Sleep deprivation
Flies were sleep-deprived for 12 hours during the dark phase using the Sleep-Nullifying Apparatus (SNAP) (47). Flies were loaded individually into 65-mm glass tubes that are placed in Drosophila Activity Monitors (TriKinetics, Waltham MA, USA). Sleep deprivation was induced by using the SNAP device to tilt activity monitors loaded with flies back and forth (−60° to 60°) every 10 s. Afterward, flies that lost at least 95% of sleep were selected for tethering. For sleep deprivation in vials, vials with 8 to 12 flies were placed on the floor of the SNAP in such a way that they would roll across the floor of the device.
Luciferase assay
Flies were fed food consisting of 1% agar, 5% sucrose, and 5 mM d-luciferin for 24 hours, after which flies were anesthetized with CO2 and had their proboscis immobilized with glue or received a sham treatment, as described. Flies were transferred to a black 96-well plate, placed under domes [c.f. (48)]. Flies are in a checkerboard pattern so flies are only in diagonally adjacent wells (i.e., up to 48 flies per plate). To correct for background noise, eight nonluciferin fed controls were added to each plate. After loading, the plate was immediately placed into a luminometer (TopCount). The plate was read approximately once every 150 s.
Proboscis immobilization
After CO2 anesthesia, the proboscis was immobilized by placing a bit of light-cured super glue (SuperGlue UV glass adhesive, 505127A, Pacer Technology, Cucamonga, CA, USA), on parts of the proboscis covering the rostrum and haustellum, but leaving the labella free, allowing flies to feed. Sham-treated controls received a bit of glue on the top of the head, covering the ocelli. Afterward, flies were given 3 days to recover, eliminating flies unable to feed.
Full-body injury assay
We performed this assay as described (30). Groups of flies were placed into a clear vial attached to a fixed spring that runs parallel to the bench surface. Pulling back the spring back to a 90° angle, then releasing it, causes the vial with flies to smash onto a rubber pad. After injury induction, flies were visually inspected for external damage. Intact flies were subsequentially tethered.
Dye injections
Microinjection pipettes (Drummond Scientific; 5 μl) were made in an electrode puller. These were placed into a syringe pump (KDS 310 Plus Nano Legacy Syringe Pump, KD Scientific). The plunger that comes with the Drummond Scientific pipettes was modified to fit the KDS 310 pump. Pipettes were loaded with mineral oil (Sigma-Aldrich). Afterward, the plunger was inserted, the pipette was placed in the pump, and the tip was broken off using tweezers. The pipette was lowered into a 200-μl Eppendorf tube containing 1% Blue #1 (31) in PBS and backfilled by slowly withdrawing the plunger. Unanesthetized flies were aspirated into a 200-μl pipette with the tip shaved off, such that the head and rostral part of the thorax emerges, effectively immobilizing the fly. The microinjection pipette was inserted into the front lateral thorax to deliver an injected volume of 50 ± 5 nl over 5 s. Flies were injected at ZT1.
Dye clearance assay
Excreted dye amounts were quantified [c.f. (31)]. Groups of 7 to 12 flies were placed into 4-ml glass scintillation vials, after being either injected or prefed with dye-colored food (Blue #1; Spectrum Chemical Manufacturing Corp., Gardena, CA). A total of 100 μl of 1% agar and 5% sucrose food was added to the cap, which was loosely screwed onto the vial to allow air into the vial. Flies were transferred to a fresh vial every hour. At the end of the experiment, 0.5-ml water was pipetted into each vial and briefly vortexed, dissolving all the blue dye. A total of 200 μl of this was pipetted into a clear 96-well plate, along with a 50% dilution series of a known quantity of dye. The amount of dye in each well is quantified by measuring its absorption of 630-nm light in a plate reader (Epoch, BioTek Instruments, Winooski, VT, USA), comparing this to the 50% dilution series (31) and dividing by the number of flies in each well to determine the average amount of dye excreted by each fly per hour.
Supplementary Material
Acknowledgments
We thank M. Kammerling, A. Augustin, and B. Shieh for assistance and R. Fleuren from Science Transmitter for assisting in the creation of the Fig. 4A and movie S6. Funding: This work was supported by the Department of the Army/USAMRAA W81XWH-16-1-0169, W81XWH-16-1-0166, and W81XWH2010211 and the Alzheimer’s Association AARG-17-532626. Author contributions: B.v.A., R.A., and B.v.S. contributed to experimental design. B.v.A., E.R.S., and M.Y. collected and analyzed data. B.v.A. and R.A. wrote the manuscript. B.v.A., R.A., and B.v.S. edited the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data are available in the manuscript or the Supplementary Materials. All data and code are available upon reasonable request.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/4/eabc2999/DC1
REFERENCES AND NOTES
- 1.Rasch B., Born J., About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besedovsky L., Lange T., Born J., Sleep and immune function. Pflugers Arch. 463, 121–137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith T. J., Wilson M. A., Karl J. P., Orr J., Smith C. D., Cooper A. D., Heaton K. J., Young A. J., Montain S. J., Impact of sleep restriction on local immune response and skin barrier restoration with and without "multinutrient" nutrition intervention. J. Appl. Physiol. (1985) 124, 190–200 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Xie L., Kang H., Xu Q., Chen M. J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D. J., Nicholson C., Iliff J. J., Takano T., Deane R., Nedergaard M., Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen N. A., Munk A. S., Lundgaard I., Nedergaard M., The glymphatic system: A beginner’s guide. Neurochem. Res. 40, 2583–2599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fultz N. E., Bonmassar G., Setsompop K., Stickgold R. A., Rosen B. R., Polimeni J. R., Lewis L. D., Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell S. S., Tobler I., Animal sleep: A review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8, 269–300 (1984). [DOI] [PubMed] [Google Scholar]
- 8.Nath R. D., Bedbrook C. N., Abrams M. J., Basinger T., Bois J. S., Prober D. A., Sternberg P. W., Gradinaru V., Goentoro L., The jellyfish Cassiopea exhibits a sleep-like state. Curr. Biol. 27, 2984–2990.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw P. J., Cirelli C., Greenspan R. J., Tononi G., Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Hendricks J. C., Finn S. M., Panckeri K. A., Chavkin J., Williams J. A., Sehgal A., Pack A. I., Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Nitz D. A., van Swinderen B., Tononi G., Greenspan R. J., Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr. Biol. 12, 1934–1940 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Dissel S., Seugnet L., Thimgan M. S., Silverman N., Angadi V., Thacher P. V., Burnham M. M., Shaw P. J., Differential activation of immune factors in neurons and glia contribute to individual differences in resilience/vulnerability to sleep disruption. Brain Behav. Immun. 47, 75–85 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stahl B. A., Slocumb M. E., Chaitin H., DiAngelo J. R., Keene A. C., Sleep-dependent modulation of metabolic rate in Drosophila. Sleep 40, zsx084 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayser M. S., Mainwaring B., Yue Z., Sehgal A., Sleep deprivation suppresses aggression in Drosophila. eLife 4, e07643 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams J. A., Sathyanarayanan S., Hendricks J. C., Sehgal A., Interaction between sleep and the immune response in Drosophila: A role for the NFκB relish. Sleep 30, 389–400 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramon F., Hernandez-Falcon J., Nguyen B., Bullock T. H., Slow wave sleep in crayfish. Proc. Natl. Acad. Sci. U.S.A. 101, 11857–11861 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raccuglia D., Huang S., Ender A., Heim M.-M., Laber D., Suárez-Grimalt R., Liotta A., Sigrist S. J., Geiger J. R. P., Owald D., Network-Specific synchronization of electrical slow-wave oscillations regulates sleep drive in Drosophila. Curr. Biol. 29, 3611–3621.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Eban-Rothschild A. D., Bloch G., Differences in the sleep architecture of forager and young honeybees (Apis mellifera). J. Exp. Biol. 211, 2408–2416 (2008). [DOI] [PubMed] [Google Scholar]
- 19.van Alphen B., Yap M. H., Kirszenblat L., Kottler B., van Swinderen B., A dynamic deep sleep stage in Drosophila. J. Neurosci. 33, 6917–6927 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yap M. H. W., Grabowska M. J., Rohrscheib C., Jeans R., Troup M., Paulk A. C., van Alphen B., Shaw P. J., van Swinderen B., Oscillatory brain activity in spontaneous and induced sleep stages in flies. Nat. Commun. 8, 1815 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.V. Dethier, The Hungry Fly (Harvard Univ. Press, 1976). [Google Scholar]
- 22.Berman G. J., Choi D. M., Bialek W., Shaevitz J. W., Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 11, 20140672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathis A., Mamidanna P., Cury K. M., Abe T., Murthy V. N., Mathis M. W., Bethge M., DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Schwarz O., Bohra A. A., Liu X., Reichert H., VijayRaghavan K., Pielage J., Motor control of Drosophila feeding behavior. eLife 6, e19892 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dijk D. J., Stanley N., Lundahl J., Groeger J. A., Legters A., Trap Huusom A. K., Deacon S., Enhanced slow wave sleep and improved sleep maintenance after gaboxadol administration during seven nights of exposure to a traffic noise model of transient insomnia. J. Psychopharmacol. 26, 1096–1107 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Berry J. A., Cervantes-Sandoval I., Chakraborty M., Davis R. L., Sleep facilitates memory by blocking dopamine neuron-mediated forgetting. Cell 161, 1656–1667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agosto J., Choi J. C., Parisky K. M., Stilwell G., Rosbash M., Griffith L. C., Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat. Neurosci. 11, 354–359 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murthy M., Turner G., Dissection of the head cuticle and sheath of living flies for whole-cell patch-clamp recordings in the brain. Cold Spring Harb. Protoc. 2013, 134–139 (2013). [DOI] [PubMed] [Google Scholar]
- 29.M. Eugenia Chiappe, V. Jayaraman, Performing electrophysiology and two-photon calcium imaging in the adult Drosophila central brain during walking behavior, in Genetically Encoded Functional Indicators, J. R. Martin, Ed. (Humana Press, 2012), vol. 72, pp. 83–101. [Google Scholar]
- 30.Katzenberger R. J., Loewen C. A., Wassarman D. R., Petersen A. J., Ganetzky B., Wassarman D. A., A Drosophila model of closed head traumatic brain injury. Proc. Natl. Acad. Sci. U.S.A. 110, E4152–E4159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shell B. C., Schmitt R. E., Lee K. M., Johnson J. C., Chung B. Y., Pletcher S. D., Grotewiel M., Measurement of solid food intake in Drosophila via consumption-excretion of a dye tracer. Sci. Rep. 8, 11536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nitabach M. N., Wu Y., Sheeba V., Lemon W. C., Strumbos J., Zelensky P. K., White B. H., Holmes T. C., Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J. Neurosci. 26, 479–489 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flood T. F., Iguchi S., Gorczyca M., White B., Ito K., Yoshihara M., A single pair of interneurons commands the Drosophila feeding motor program. Nature 499, 83–87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dow J. A. T., Davies S. A., The Drosophila melanogaster malpighian tubule. Adv. Insect Phys. 28, 1–83 (2001). [Google Scholar]
- 35.Iliff J. J., Wang M., Liao Y., Plogg B. A., Peng W., Gundersen G. A., Benveniste H., Edward Vates G., Deane R., Goldman S. A., Nagelhus E. A., Nedergaard M., A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandes C., Plautz J. D., Stanewsky R., Jamison C. F., Straume M., Wood K. V., Kay S. A., Hall J. C., Novel features of Drosophila period transcription revealed by real-time luciferase reporting. Neuron 16, 687–692 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Itskov P. M., Moreira J.-M., Vinnik E., Lopes G., Safarik S., Dickinson M. H., Ribeiro C., Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 5, 4560 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blum A. L., Li W., Cressy M., Dubnau J., Short- and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Curr. Biol. 19, 1341–1350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.M. Demerec, Biology of Drosophila (John Wiley and Sons, 1965). [Google Scholar]
- 40.Lehmann F.-O., Heymann N., Unconventional mechanisms control cyclic respiratory gas release in flying Drosophila. J. Exp. Biol. 208, 3645–3654 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Zhang S. L., Yue Z., Arnold D. M., Artiushin G., Sehgal A., A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173, 130–139.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Artiushin G., Zhang S. L., Tricoire H., Sehgal A., Endocytosis at the Drosophila blood-brain barrier as a function for sleep. eLife 7, e43326 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He J., Hsuchou H., He Y., Kastin A. J., Wang Y., Pan W., Sleep restriction impairs blood-brain barrier function. J. Neurosci. 34, 14697–14706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh P., Donlea J. M., Bidirectional regulation of sleep and synapse pruning after neural injury. Curr. Biol. 30, 1063–1076.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanhope B. A., Jaggard J. B., Gratton M., Brown E. B., Keene A. C., Sleep regulates glial plasticity and expression of the engulfment receptor draper following neural injury. Curr. Biol. 30, 1092–1101.e3 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Brembs B., Operant learning of Drosophila at the torque meter. J. Vis. Exp. , 731 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw P. J., Tononi G., Greenspan R. J., Robinson D. F., Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature 417, 287–291 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Sharp B., Paquet E., Naef F., Bafna A., Wijnen H., A new promoter element associated with daily time keeping in Drosophila. Nucleic Acids Res. 45, 6459–6470 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/4/eabc2999/DC1


