Fig. 3. OTUD5 regulates CNS precursor and neural crest cell differentiation via its K48-ubiquitin chain–specific deubiquitylation activity.
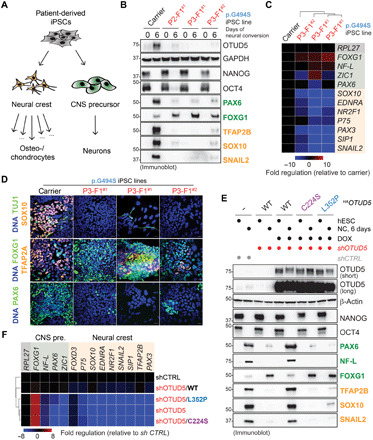
(A) Schematic overview of the neural conversion paradigm. (B) Reduction of OTUD5 levels causes aberrant neural conversion. iPSCs derived from OTUD5 p.Gly494Ser patients or the maternal carrier were subjected to neural conversion for 6 days. Differentiation was monitored by immunoblotting using indicated antibodies against hESC, CNS precursor, and neural crest markers. (C) Same experimental approach as described in (B), but cells were analyzed by qRT-PCR for expression of CNS precursor markers (green) and neural crest markers (orange). Marker expression was normalized to carrier control followed by hierarchical clustering. RPL27, endogenous control. (D) Same experimental approach as described in (B), but cells were subjected to neural conversion for 9 days and analyzed by immunofluorescence microscopy using antibodies against indicated CNS precursor and neuronal markers (green) or neural crest markers (orange). Scale bars, 20 μm. (E) K48-ubiquitin chain–specific deubiquitylation activity of OTUD5 is required for proper CNS precursor and neural crest differentiation. hES H1 cells stably expressing shRNA-resistant and doxycycline-inducible WT, catalytically inactive (C224S), or K48 chain cleavage–deficient (L352P) HAOTUD5 were generated. Cells were depleted of endogenous OTUD5 using shRNA as indicated, treated with or without doxycycline (DOX), and subjected to neural conversion (NC) for 6 days. This was followed by immunoblotting using the indicated antibodies against hESC, CNS precursor, and neural crest markers. (F) Same experimental approach as described in (E), but cells were analyzed by qRT-PCR analysis for expression of CNS precursor markers (green) and neural crest markers (orange). Marker expression was normalized to shcontrol followed by hierarchical cluster analysis. RPL27, endogenous control.
