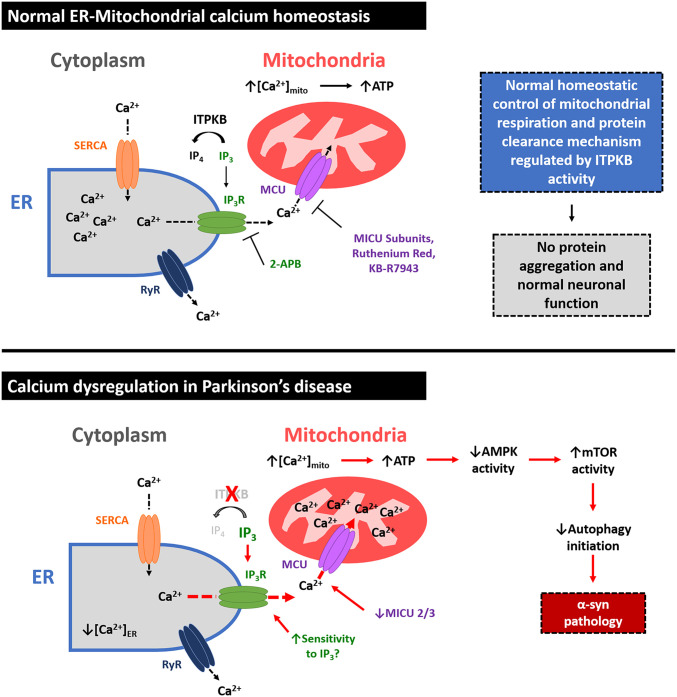
Fig. 6.
Cellular model for ITPKB function in PD. In healthy neurons (Top), the ER is the predominant storage organelle for intracellular calcium (Ca2+), which is transiently released in response to various upstream signaling events to mediate diverse cellular functions. ER Ca2+ can be released through either the IP3R or the Ryanodine receptor (RyR), while the ER-cytosol Ca2+ gradient is preserved by SERCA. In response to increases in intracellular IP3 levels, Ca2+ is released from the ER via IP3Rs, leading to Ca2+ accumulation in mitochondria mediated by transport into the inner mitochondrial matrix via the mitochondrial calcium uniporter (MCU). Transient increases in mitochondrial calcium levels ([Ca2+]mito) drive the production of ATP from ADP by oxidative phosphorylation. ITPKB enzymatic activity negatively regulates IP3-mediated ER calcium release by converting IP3 to IP4, thereby protecting cells from overproduction of ATP and accumulation of ROS. The MCU subunits MICU1, MICU2, and MICU3 serve as Ca2+ sensors to control homeostatic levels of Ca2+ import into mitochondria. In PD (Bottom), loss of ITPKB activity, changes in MCU regulatory subunit composition, and/or chronic increases in intracellular IP3 levels or sensitivity of IP3Rs to IP3 may contribute to increased [Ca2+]mito, leading to ROS generation, mTOR activity, and the accumulation of misfolded proteins, including α-syn aggregates. Pharmacological agents that positively modulate ITPKB activity or inhibit ER-to-mitochondria calcium exchange may represent a new therapeutic approach for the treatment of synucleinopathies such as PD.