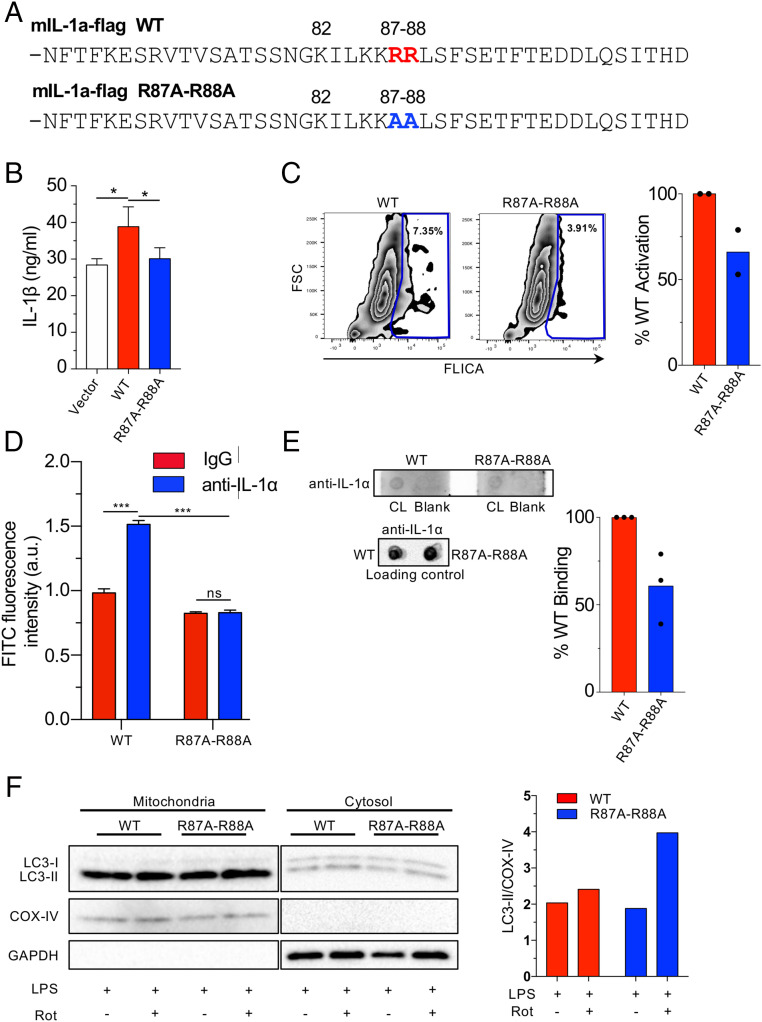
Fig. 7.
Mutated pro-IL-1α reduces IL-1β secretion and CL binding. (A) Mouse pro-IL-1α mutation construct. Changes are indicated. (B and C) Il1a−/− BMDMs were reconstituted with WT or R87A-R88A mouse Il1a and primed with LPS (6 h), then treated with ATP (45 min). IL-1β secretion measured by ELISA (B) and caspase-1 activity was measured by FLICA using flow cytometry (C). (D) HEK293 cells were transfected with mIL-1a-WT-Flag or mIL-1a-R87A-R88A-Flag. Anti-FLAG beads were used to immunoprecipitate the FLAG-proteins. The beads were then incubated with FITC labeled CL and fluorescence was measured using a plate reader. (E) Membrane lipid strips were incubated with cell lysates from immortalized Il1a−/− macrophages expressing flag-tagged WT and R87A-R88A pro-IL-1α, then washed and developed with an anti–IL-1α antibody. Densitometry of pro-IL-1α binding to CL was performed and normalized to total pro-IL-1α in the loading control. (F) Il1a−/− BMDMs were reconstituted with WT or R87A-R88A mouse Il1a and were treated 50 nM bafilomycin A1 for 1 h, then stimulated with LPS for 3 h and treated with Rotenone (1 μM) for 15 min. Immunoblots for LC3, COX-IV, and GAPDH in cytosolic and mitochondrial fractions are shown. Densitometry of LC3-II normalized to COX-IV is shown (mitochondrial fraction). Data shown are representative from two (C, D, and F) to three (B and E) experiments. All data represent mean ± SD. One-way ANOVA with Tuckey’s posttest (B) or two-way ANOVA with Bonferonni’s posttest (D) was used for statistical analyses. *P < 0.05, ***P < 0.001.