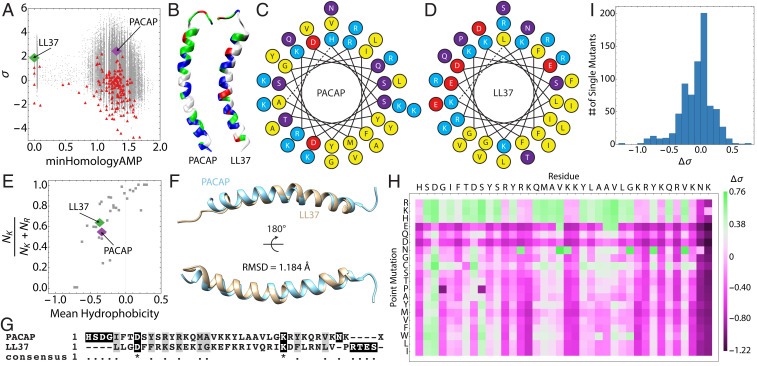
Fig. 1.
PACAP is a GPCR-binding neuropeptide with strong physicochemical homology to human cathelicidin from innate immunity. (A) Screening a neuropeptide database (red) using the support vector machine-based MAPT for sequences with hidden membrane-restructuring activity revealed PACAP (purple) to be a hypothesized endogenous HDP. LL37 (green) is shown for comparison alongside sequences generated by a Monte Carlo–based directed search of sequence space (gray). (B) Comparison of the distribution of residue character along the lengths of PACAP and LL37 (blue = cationic, green = hydrophilic, white = hydrophobic, red = anionic). Helical wheel diagrams of (C) PACAP and (D) LL37 show similar distributions of hydrophobic (yellow) and cationic residues (teal). Anionic amino acids (red) and polar amino acids (purple) are also shown. (E) PACAP and LL37 exhibit similar lysine-to-arginine ratios and mean hydrophobicities, which suggests that PACAP (purple) can generate negative Gaussian membrane curvature similar to LL37 (green) and other ɑ-helical AMPs (gray). (F) Structural alignment of PACAP and LL37 yields an rmsd of 1.184 Å, demonstrating close structural similarity, despite (G) very low sequence homology. (H and I) An in silico mutational analysis of the entire PACAP sequence demonstrates that PACAP is locally optimized for membrane-remodeling activity, and that the majority of mutations reduce the microbicidal potential of the peptide.