Fig. 5.
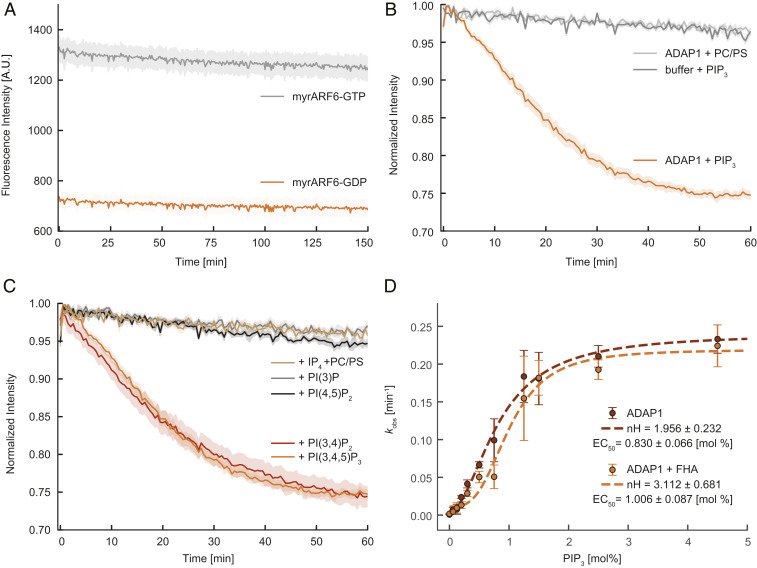
ADAP1 is a PIP3/PI(3,4)P2 membrane-dependent GAP whose activity is influenced by KIF13B. (A) Intrinsic tryptophan fluorescence of myrARF6 (4.5 µM) preloaded with GTP (gray) or GDP (orange) over time. (B) Time course of normalized tryptophan fluorescence of 4.5 µM myrARF6-GTP in the absence (dark gray) or presence of 1.5 µM ADAP1 with either 375 µM vesicles containing no PIP3 (light gray) or 2.5% PIP3 vesicles (orange). (C) Normalized tryptophan fluorescence of 4.5 µM myrARF6-GTP in the presence of 1.5 µM ADAP1 and 375 µM vesicles with either 2.5% PIP3, PI(3,4)P2, PI(3)P, PI(4,5)P2, or vesicles without phosphoinositides together with 20 µM IP4. Data for the PIP3 condition are the same for B and C. (D) Measurement of the catalytic activity of 1.5 µM ADAP1 (kobs) with 4.5 µM myrARF6-GTP as a function of PIP3 content in vesicles (375 µM) in the absence (red) or presence of 10 µM KIF13B’s FHA domain (orange). kobs values were fitted to a Hill equation (dashed lines) extracting the Hill coefficients of 1.96 (± 0.23) in the absence and 3.11 (± 0.68) in the presence of the FHA domain. Errors are SEMs for A–C and SDs for D and are derived from at least three independent measurements per condition.