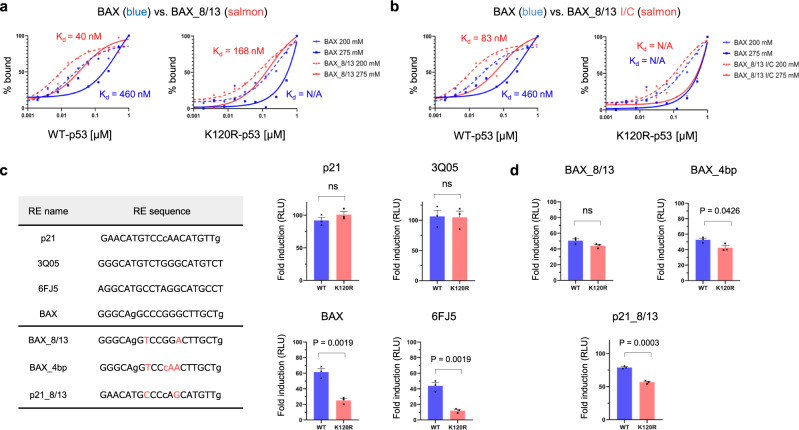
Fig. 4. Rewriting the RE code reprograms p53 function in vitro and in cells.
a G/C to A/T conversions at positions 8 and 13 in BAX RE increase its affinity for WT p53 (from 460 nM to 40 nM, left) and restore K120R-p53 binding (right). b G/C to I/C conversions at positions 8 and 13 in BAX RE increase its affinity for WT p53 (from 460 nM to 83 nM, left), but do not have an impact on K120R-p53 binding deficiency (right). Data are shown as graphs depicting the percent of bound protein and plotted as a function of the protein concentration (from 0.001 to 1 μM, in log scale). Two independent measurements were performed at “low-salt” (dashed curves) and “high-salt” (solid curves) buffer conditions. Relevant Kd values measured at high-salt concentrations are depicted on the graphs. I/C inosine/cytosine. N/A not available. c A luciferase reporter plasmid containing p21, 3Q05, 6FJ5, BAX, BAX_8/13, BAX_4bp or p21_8/13 RE (nucleotide sequences are listed in table) were co-transfected with an empty vector, WT or K120R-p53 expression plasmid. Twenty-four hours after transfection cells were harvested and luminescence was measured. K120R-p53 has identical activity as WT p53 protein on A/T-rich p21 and 3Q05 REs, but is defective in trans-activating G/C-rich BAX and 6FJ5 REs. These data confirm K120R-p53 binding behavior observed in vitro. Data are presented as a fold change of measured luminescence in either WT (blue) or K120R (salmon)-p53-expressing cells over luminescence measured in cells transfected with an empty vector (no p53). d Substituting G/C to A/T bps in BAX RE (“BAX_8/13”, converted nucleotides are depicted in salmon letters) or changing positions 8–13 from CCCGGG to TCCCAA (“BAX_4bp”) restores K120R-p53 transactivation activity. Conversely, substituting A/T to G/C bps in p21 RE (“p21_8/13”) reintroduces K120R deficiency. Graphs in panels c and d show cumulative data from three independent experiments (mean ± s.e.m.). Two-tailed unpaired Students t-test was used for statistical analysis in panels c and d. RLU, relative light units. Source data are provided as a Source Data file.