Abstract
We aimed to estimate metabolic bone profile in a large cohort of healthy, adult Indian population to generate reference standards of serum calcium, phosphate and alkaline phosphatase (ALP), 25 (OH) Vitamin D and iPTH, and also to find out the prevalence of Vitamin D deficiency in healthy population. Apparently healthy people in the age group of 20–80 years, residing in the union territory of Chandigarh were chosen. Fasting samples for serum calcium, phosphate, albumin, alkaline phosphatase (ALP), 25 (OH) D and iPTH were collected and were processed on the same day. We recruited 930 healthy subjects from different subsectors of Chandigarh. Final analysis was done for 915 subjects. Out of this, 530 (58%) were women and 385 (42%) were men. The study participants were divided into two groups, less than and more than 50 years for the men and pre and post-menopausal for the women. The serum calcium, phosphate, ALP and iPTH were significantly higher in the post-menopausal women compared to the pre-menopausal women. The median plasma 25 (OH) D in men and women was 12.5 ng/mL and 14.3 ng/mL, respectively. 25 (OH) D deficiency was seen in 65.4% of individuals. 25 (OH) D levels co-related negatively with iPTH levels (r = − 0.4, p < 0.0001), and showed an increasing trend with age. We have thus presented metabolic bone profile of healthy, adult north Indian population. These reference values can be used for diagnosis and monitoring of various MBDs. Vitamin D deficiency is still rampant in our population in spite of increasing awareness.
Keywords: Calcium, Vitamin D, Phosphate, ALP
Introduction
Metabolic bone disease (MBD) is the third most common cause of endocrine disorders [1]. It includes a wide spectrum of disorders which encompasses rickets, osteomalacia, osteoporosis and primary hyperparathyroidism. Alterations in calcium-phosphate homeostasis, disorders of bone mineralization, and abnormalities of bone mass form the pathophysiological basis of metabolic bone diseases. Prevention, early diagnosis, and timely treatment can reduce morbidity and mortality related to MBD. Diagnosis of MBD requires a battery of tests like serum calcium profile [serum calcium, phosphate, albumin, and alkaline phosphatase (ALP)], 25 hydroxy vitamin D [25 (OH) D], parathyroid hormone (iPTH), skeletal survey, bone histology and molecular (genetic) workup [2]. Out of these, serum calcium profile is the preliminary investigation and is often alone able to clinch a majority of the diagnosis related to MBD.
The calcium profile and normal reference range parameters are dependent upon nutrition and vitamin D status as well as the method of measurement. In addition to this, calcium profile needs to be revised periodically with the changing nutritional and vitamin D status of the community. The normative data of the metabolic bone profile is available for the pediatric population from India. To the best of our literature search, well-planned and dedicated studies to generate normative data for metabolic bone profile for healthy, adult Indian population are scarce.
Given this scarcity of data on metabolic bone profile, this present study was planned, to estimate metabolic bone profile in a large cohort of the healthy, adult Indian population in Chandigarh to generate reference standards of serum calcium, phosphate, alkaline phosphatase (ALP), 25(OH) D and iPTH. We also wished to see the change in trends of these parameters with age, menopausal status and between the genders.
The population of the Union territory of Chandigarh according to 2011 Census is 10,54,686. It is a growing economy, with the population density having increased nine-folds in the last 5 decades. The per capita income of Chandigarh is Rupees 67,370 which is the highest in the country. We can extrapolate these facts to believe the living standards and nutritional status of Chandigarh are optimum for a demographic study involving healthy individuals to be planned here. In our study, we recruited individuals who were either natives of Chandigarh or had been residing in Chandigarh for at least the last 5 years.
Materials and Methods
In this cross-sectional study, healthy, adult volunteers were recruited from the community (from Dec 2016 till May 2018), as a part of the Chandigarh Urban Bone Epidemiological Study (CUBES).The sample size was calculated following the recommendations of the Clinical Laboratory Standards Institute (CLSI) [3],with a power of study more than 80%. Sample size thus came out be 915. All selected participants were duly pre-informed about the study and written informed consent was taken from each participant. The study was approved by the institute ethics committee.
Recruitment of Volunteers
Healthy people in the age group of 20–80 years were chosen. These individuals were chosen from 4 sectors from within the Union Territory of Chandigarh. Sectors were chosen randomly, by lottery method, and houses within each sector were chosen by systematic random sampling, by selecting every fifth house from a random starting point. ‘Kish method’ was used to select members from within each household, from amongst the members who were fulfilling the inclusion criteria [4]. This method uses a pre-assigned table of random numbers to find the person to be interviewed. The participants were recruited in equal number from each sector. A pre-formed proforma comprising of questions on demography, personal history, history of intake of milk and milk-based products, history of medications including vitamin D supplements was filled for the chosen individual, via a house-to-house visit. Biochemical investigations including renal function test, liver function test, HbA1c, and thyroid profile were planned for each individual. Those individuals who had chronic systemic illnesses such as diabetes mellitus, chronic renal and liver disease, or endocrine diseases like hyperthyroidism, primary hyperparathyroidism were excluded from the study. Also, those who had a current fracture or were on medications likely to affect bone health were excluded.
Collection of Samples
The recruited volunteers were given a reminder telephonically to remain fasting a night before the day of sampling. Fasting samples were collected between 7 and 8 am. Samples for serum calcium, phosphate, albumin and alkaline phosphatase (ALP) were collected in plain vials. Sample for 25(OH) D was collected in EDTA vials and sample for iPTH was collected in pre-chilled EDTA vials which were placed and transported in the cold chain. Samples were processed on the same day at Endocrinology and Biochemistry laboratories of Postgraduate Institute of Medical Education and Research, Chandigarh, India. Serum calcium, albumin, phosphorus and ALP levels were assessed by random access autoanalyzer (Beckman coulter 5811).
The reference range for calcium as per the kit is 8.6–10.3 mg/dL. The intra assay coefficient of variation (CV) was 1.34%. The reference range for albumin is 3.5–5.7 g/dL. The intra assay CV for albumin was 1.5%. The reference range for phosphorus is 3.7–7.2 g/dL. The intra assay CV for phosphorous was 1.5%. The reference range for ALP is 34–104 U/L. The intra assay CV for ALP was 1.5%.
Plasma iPTH and 25(OH) D were measured by chemiluminescence assay using commercially available kits (Elecsys 2010 system, Roche diagnostic, Switzerland). Vitamin D deficiency was defined as plasma 25(OH) D levels < 20 ng/mL, insufficiency as plasma 25(OH) D levels between 20 and 30 ng/mL and sufficiency as plasma 25(OH) D levels > 30 ng/mL [5].
Statistical Analysis
GraphPad Prism 5 was used for data analysis. Kolmogorov–Smirnov test was applied to check for normality of data. Student T test was used to compare the means of two groups for parametric data and Mann–Whitney U test for non-parametric data. ANOVA was used for comparing means of more than two groups for parametric data and Krusal–Wallis test was used for non-parametric data. Spearman test was used to compute nonparametric co-relation and Pearson test was used to compute parametric co-relation.
Results
Demography
We recruited 930 healthy subjects from different subsectors of Chandigarh. The final analysis was done for 915 subjects, as 5 samples were found to be hemolysed, 7 individuals had deranged renal or liver function tests, and 3 individuals were detected to be diabetic. Out of this, 530 (58%) were women and 385 (42%) were men. The median age of the recruited population was 41 years, with the median age of the men being 40 years while that of the women being 42.5 years. The median height of the population was 158 cm. The median height of the men was 168.0 cm while that of the women was 153 cm. The median BMI of the population was 26.1 kg/m2. The median BMI of the men was 25.8 kg/m2 and that of the women was 26.4 kg/m2, with no statistically significant difference between the two.
The study participants were divided into two groups; less than and more than 50 years of age (men) and pre and post-menopausal (women). Out of the 530 women, 350 were pre-menopausal while 180 were post-menopausal. Out of the 385 men, 249 were younger than 50 years while 136 were older than 50 years of age.
Use of vitamin D supplements was seen in 130 (14.2%) subjects. The frequency of use of vitamin D supplements was higher in women and older age groups (p value < 0.03). Consumption of milk and milk products was seen in 540(59.2%) subjects.
Serum Calcium
The medianserum calcium in men was 8.9 mg/dL (range 7.3–9.9 mg/dL) and that in the women was also 8.9 mg/dL (range 7.6–9.9 mg/dL).The median calcium levels in pre and post-menopausal women were 8.9 mg/dL and 9 mg/dL, respectively, while amongst the men these values were 8.8 mg/dL and 8.9 mg/dL, for men aged less than and more than 50 years, respectively. The calcium levels were significantly higher in the post-menopausal women as compared to the pre-menopausal women (p 0.024), while the calcium levels did not differ significantly between the two age groups among the men.
Across all age-groups, none of the participants had hypercalcemia (serum calcium ≥ 10.4 mg/dL). Among the men, the serum calcium levels co-related negatively with age, but the co-relation was weak (r = − 0.018), while among the women, serum calcium levels co-related positively with age (r = 0.14). Serum calcium levels had a statistically significant weak positive correlation with BMI (r = 0.240).
Serum Phosphate
The median serum phosphate in men was 3.6 mg/dL (range 2.2–6 mg/dL) and that in the women was also 3.6 mg/dL (range 2.1–5.3 mg/dL).The median phosphate levels in pre and post-menopausal women were 3.6 mg/dL and 3.7 mg/dL, respectively, while these values were 3.7 mg/dL and 3.4 mg/dL, for men aged less than and more than 50 years, respectively. The phosphate levels were significantly higher in the post-menopausal women as compared to the pre-menopausal women (p 0.004), while the phosphate levels were significantly lower in the older men as compared to the younger ones (p < 0.0001). Among the men, the serum phosphate level co-related negatively with age (r = − 0.2), while in the women, it co-related positively with age (r = 0.06).
Serum Alkaline Phosphatase (ALP)
The median serum ALP in men was 102 IU/L (range 31–259 IU/L) and that in the women was also 102 IU/L (range 31–259 IU/L). The median ALP levels in pre and post-menopausal women were 98 IU/L and 107 IU/L, respectively, while these values were 102 IU/Land 98 IU/L, for men aged less than and more than 50 years, respectively. The ALP levels were significantly higher in the post-menopausal women as compared to the pre-menopausal women (p 0.02), while the ALP levels did not differ significantly in the two age groups among the men.
Among the men, serum ALP levels co-related negatively with age,but the co-relation was weak (r = − 0.08),while in the women, it co-related positively with age (r = 0.16). Also, among both the men and women, serum calcium correlated positively with serum ALP (r = 0.12, p < 0.01 and r = 0.09, p < 0.02 respectively).
Plasma 25(OH) D
The median plasma 25(OH) D level was 13.5 ng/mL (range 3–70 ng/mL). The median plasma 25(OH) D in men was 12.5 ng/mL (range 3–70 ng/mL) while that in the women was 14.3 ng/mL) (range 3–70 ng/mL). However, the difference was statistically in significant (p 0.13).The median 25 (OH) d Levels in pre and post-menopausal women were 15.1 ng/mL and 12.4 ng/mL respectively, while these values were 11.4 ng/mL and 17.7 ng/mL, for men aged less than and more than 50 years respectively. The 25 (OH) d Levels were significantly higher in the pre-menopausal women as compared to the post-menopausal women (p 0.0006), while they were significantly higher in the older men as compared to the younger men (p 0.004).
The median value of plasma 25(OH) D among those not taking vitamin D supplements was 16.9 ng/mL, while among those taking vitamin D supplements was 28.9 ng/mL, with the difference being statistically significant (p < 0.0001). Likewise, the median plasma iPTH, calcium, phosphate and ALP among those individuals not taking vitamin D supplements were 50 pg/mL, 8.9 mg/dL, 4 mg/dL, and 121 IU/L, respectively. The corresponding values in those who were on vitamin D supplements were 42.7 pg/mL, 8.8 mg/dL, 3.6 mg/dL, and 108.3 IU/L, respectively, with only the difference in iPTH being statistically significant (p < 0.005).
25(OH) D deficiency was seen in 599 (65.4%) of individuals. Of these 599 individuals, 262 (43.7%) were men and 337 (56.3%) were women. Vitamin D insufficiency and normal levels were seen in 138 (15%) and 178 (19.4%) individuals respectively.
Plasma iPTH
The median iPTH levels were 44.1 pg/mL (range 11–222 pg/mL). The median plasma iPTH in men was 43.6 pg/mL (range 11.5–165.3 pg/mL).MedianiPTH levels in the women were 45.4 pg/mL (range 11–222 pg/mL). However, the difference was statistically insignificant (p < 0.06) as shown in Table 1. The median PTH levels in pre and post-menopausal women were 43.2 pg/mL and 46.6 pg/mL respectively, while these values were 52.0 pg/mL and 35.6 pg/mL, for men aged less than and more than 50 years respectively. The PTH levels were significantly higher in the post-menopausal women as compared to the pre-menopausal women (p 0.02), while they were significantly higher in the younger men as compared to the older men (p 0.0001) 25(OH) D levels co-related negatively with iPTH levels (r = − 0.4, p < 0.0001) (Fig. 1).
Table 1.
Biochemical parameters in the study population
| Men (< 50 years) | Men (> 50 years) | Pre-menopausal women | Post-menopausal women | |
|---|---|---|---|---|
| N | 249 | 136 | 350 | 180 |
| Calcium (mg/dL) | ||||
| Median | 8.8 | 8.9 | 8.9 | 9.0 |
| IQR* | 8.5–9.2 | 8.7–9.1 | 8.6–9.2 | 8.7–9.3 |
| Phosphorous (mg/dL) | ||||
| Median | 3.7 | 3.4 | 3.6 | 3.7 |
| IQR | 3.3–4.1 | 3.1–3.8 | 3.2–3.9 | 3.4–4.1 |
| ALP (IU/L) | ||||
| Median | 102 | 98 | 98 | 105 |
| IQR | 81–120 | 78–115 | 79–122 | 84–125 |
| 25 (OH) Vitamin D (ng/mL) | ||||
| Median | 11.4 | 17.7 | 15.1 | 12.4 |
| IQR | 7–21.8 | 7.2–34.4 | 8.2–28.3 | 6.7–19.3 |
| PTH (pg/mL) | ||||
| Median | 52.0 | 35.6 | 43.2 | 46.6 |
| IQR | 36.3–68.9 | 25.4–50.4 | 31.5–58.3 | 35–59 |
*Inter-quartile range
Fig. 1.
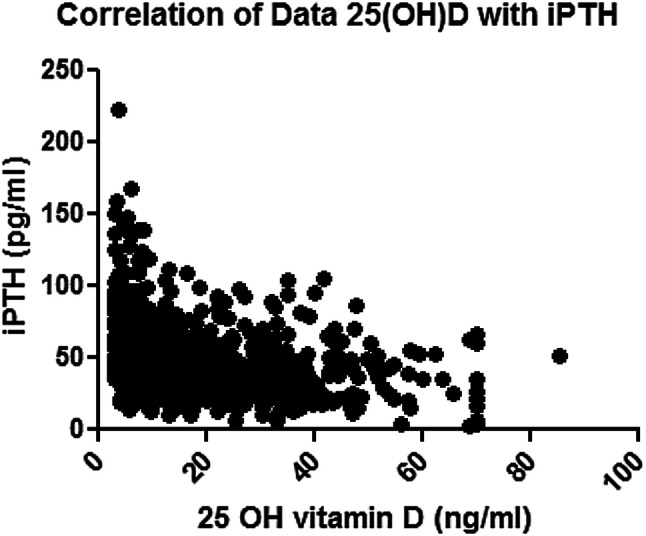
Scatter diagram depicting the inverse correlation between serum 25 (OH) D (on the X axis) and serum iPTH (on the Y axis)
As per ROC curve, iPTH value of 43.37 pg/mL predicted vitamin D deficiency at 20 ng/mL with a sensitivity of 62.4% and specificity 65.8% (Fig. 2).
Fig. 2.
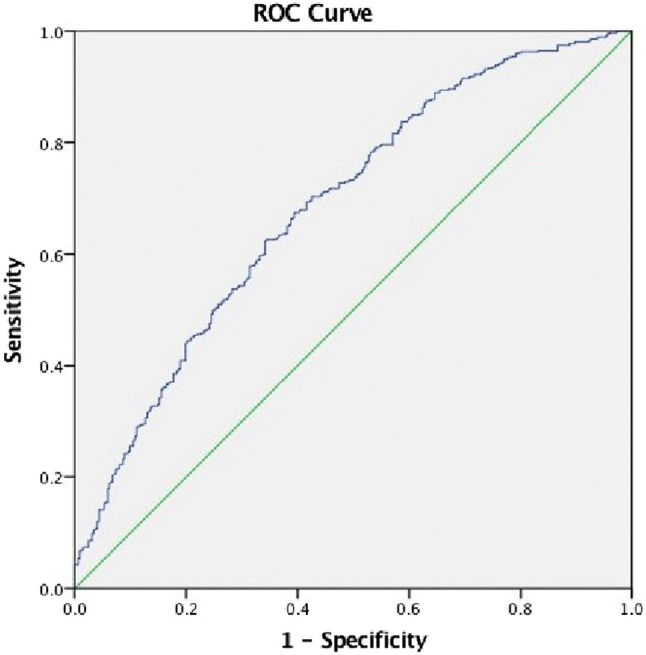
ROC curve: iPTH of 43.375 pg/mL predicted vitamin D deficiency at 20 ng/mL with a sensitivity of 62.4% and a specificity 65.8%
Serum calcium levels had a statistically significant weak positive correlation with 25(OH) D levels (r = 0.128) and a weak negative correlation with iPTH levels (r = − 0.401). Among the men, serum phosphate levels did not correlate with 25(OH) D levels, however, among the women, there were a significant correlation between serum phosphate and 25(OH) D levels (r = 0.01, p < 0.01). There was no correlation between serum ALP and 25(OH) D levels in the men, while in the women, there was a negative correlation between the two (r = − 0.1, p < 0.001).Vitamin D levels correlated positively with age though weakly (r = 0.248) suggesting that with increasing age there was an increase in vitamin D levels. 25(OH) D levels were higher in those on vitamin D supplements compared to those who were not (p < 0.02).
The median values and inter-quartile ranges (IQR) for the various parameters are depicted in Table 1.
Discussion
We have presented normative data of serum calcium, phosphate, ALP, 25(OH) D levels and iPTHin adult healthy population (n = 915) of north India (Chandigarh: latitude 30°44′10″ longitude 76°47′18″) and have generated reference ranges for the metabolic bone profile for the first time in a single study. Such data are useful for diagnosis and monitoring of various metabolic bone disorders (hypophosphatasia, hypophosphatemic osteomalacia, and chronic kidney disease) in different age groups. We observed that Vitamin D deficiency is still very high in untreated healthy individuals. We also collected data on the use of vitamin D supplements among the healthy population and have estimated serum 25(OH) D levels and iPTH levels in them. There has been no study from India which has assessed the over the counter use of vitamin D and calcium supplements in general population. Our study shows higher use of such supplements in the older population and women.
As per ROC curve, iPTH value of 43.37 pg/mL predicted vitamin D deficiency at 20 ng/mL with a sensitivity of 62.4% and specificity 65.8%.
Median calcium was 8.9 mg/dL both in the men and women. This was slightly less as compared to the study by Marwaha et al. where the mean calcium was 9.5 ± 0.4 mg/dL in men and 9.6 ± 0.4 mg/dL in women [6]. However, in that study, only individuals aged > 50 years were recruited. In another multicentre study from India designed to study peak BMD among individuals aged 20–29 years, the mean calcium among men was 9.7 ± 0.8 mg/dL while that among women was 9.6 ± 0.7 mg/dL.
In our study, we observed that the serum calcium was higher in post-menopausal women, which is probably reflective of the high prevalence of over-the-counter intake of calcium and Vitamin D supplements among the elderly population, and possibly also because of increasing adiposity with age, as calcium levels were co-related positively to the BMI. Usually, a decline in serum calcium is expected in post-menopausal women as estrogen deficiency leads to decreased intestinal calcium absorption and decreased renal calcium conservation.
Various other Indian studies have also assessed calcium status in pre-menopausal v/s post-menopausal women. In the study by Meena Desai et al. mean serum calcium levels were lower in the post-menopausal women [7]., while the study by Muni Radha Jadaa et al. [8] showed slightly higher mean serum calcium among postmenopausal group compared to premenopausal women.
A similar observation was noted in the Tromso study, wherein calcium levels were stable in the pre-menopausal women but increased slightly in the post-menopausal age group, and showed a positive co-relation with BMI and coffee consumption [9].
The median serum phosphate in both men and women was 3.6 mg/dL. In the aforementioned study by Marwaha et al. the mean serum phosphate in men was 3.4 ± 0.4 mg/dL while that in women was 3.7 ± 0.4 mg/dL [6] In the ICMR study, mean phosphate among men and women was 4.1 ± 0.7 mg/dL and 4.1 ± 0.8 mg/dL respectively [10]. Serum phosphate levels co-related negatively with age in men and showed a decreasing trend with advancing age, but such a trend was not seen among the women. Similar results have been seen in previous epidemiological studies as well [11]. The decline in serum phosphorous with advancing age can be attributed to the decreased phosphate reabsorption in the proximal convoluted tubules of kidneys with age, while it is not so evidently seen in women due to a transient increase in serum phosphate after menopause.
The median serum ALP both in men and womenwas 102 IU/L. In the study by Marwaha et al., the mean serum ALP in men was 203.0 ± 61.0 IU/L while that in women was 236.0 ± 73.0 IU/L [6].We noted that ALP was significantly higher in post-menopausal women as compared to pre-menopausal women. This increase in ALP with increasing age is expected as the bone turnover increases in the post-menopausal years. Also, the lower vitamin D levels in the post-menopausal women can explain the higher ALP levels, as bone turnover is expedited in the face of low vitamin D. Similar to ALP; PTH was also higher in the post-menopausal women as compared to the pre-menopausal women. This is expected as just like ALP, PTH is also a marker of bone turnover.
In our study, the 25(OH) D was lower in the elderly (post-menopausal) women, as compared to the younger women, likely as a result of higher consumption of vitamin D supplements among, while it was vice versa among the men. Overall, vitamin D deficiency was seen in 599 (65.4%) of individuals. Of these 599 individuals, 262 (43.7%) were men and 337 (56.3%) were women. The mean 25(OH) D levels in the study by Marwaha et al. was 9.8 ± 7.9 ng/mL among the women and 9.7 ± 6.8 ng/mL among the men, with the mean iPTH levels being 61.1 ± 39.4 pg/mL and 61.0 ± 41.9 pg/mL respectively. Their mean serum vitamin D was lower than what was seen in our study and that can explain the higher ALP levels in their study.
In one of the earlier studies on global vitamin D status and hypovitaminosis D, serum 25(OH) D levels below 25 ng/mL were prevalent in all regions studied, while < 10 ng/mL were more common in south Asia and middle east [12] In a study from south India, vitamin D deficiency (< 20 ng/mL) was seen in as high as 53% of the healthy population [13]. In a study by Marwaha et al. from Delhi, the prevalence of vitamin D deficiency was much higher at 91.2% among the healthy population [6]. In another multicenter study from India, the mean serum 25(OH) D levels in men and women were observed to be 16.9 ± 10.5 and 13.6 ± 10.2 ng/mL respectively, again falling in the deficient category [10] In our study, in the 20–29 year age group, mean serum 25(OH) D was 14.5 ± 14.8 ng/mL among the men and 12.2 ± 8.5 ng/mL among the women. The prevalence of 25 (OH) D deficiency is lower in our study compared to previous studies, likely due to increasing awareness and increasing over the counter use of vitamin D supplements.Serum 25(OH) D levels of > 70 ng/mL were seen in 7 men and 6 women. All of them were on vitamin D supplements.
Most of the studies have shown higher levels in women and younger age groups which is contrasting to our study. This high prevalence of vitamin D deficiency seen in our study as well as other studies from India can probably be attributed to multiple factors. Darker skin in our population hinders the cutaneous synthesis of vitamin D. Also, clothing practices, increasing urbanization leading to maximum time being spent indoors both at work and at home and increasing use of sunscreen creams are also responsible for it.
Limitations and Strength
The present study recruited participants from Chandigarh (North India) only, so results may not be fully applicable to throughout India. However, our study has robust randomization and we have also performed a complete bone metabolic profile for the adult population (20–80 years of age).
Conclusion
We have provided a complete metabolic bone profile of healthy, adult north Indian population. These reference values can be used for diagnosis and monitoring of various MBDs. Vitamin D deficiency is still rampant in our population in spite of increased awareness. However, over-zealous use of supplements/over the counter use of vitamin D supplements is also an emerging problem.
Funding
None received.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflicts of interest to report.
Research Involving Human Participants and/or Animals
The research involves human volunteers.
Informed Consent
Written informed consent was taken from all the participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anshita Aggarwal, Email: anshilh10@gmail.com.
Sant Ram, Email: drsantram2016@gmail.com.
Abhilasha Garg, Email: garg_abhimansa@yahoo.co.in.
Rimesh Pal, Email: rimesh.ben@gmail.com.
Anil Bhansali, Email: anilbhansaliendocrine@gmail.com.
Priyanka Singh, Email: priyankasingh3009@gmail.com.
Sadhna Sharma, Email: sadhnabiochem@gmail.com.
J. S. Thakur, Email: jsthakur64@gmail.com
Naresh Sachdeva, Email: naresh_sach@rediffmail.com.
Sanjay Kumar Bhadada, Email: bhadadask@gmail.com, Email: bhadadask@rediffmail.com.
References
- 1.Bhansali A. Metabolic bone disease: newer perspectives. Indian J Endocrinol Metab. 2012;16(Suppl 2):S140–S141. doi: 10.4103/2230-8210.104023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane NE. Chapter 101—metabolic bone disease. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, editors. Kelley and Firestein’s textbook of rheumatology. 10. Amsterdam: Elsevier; 2017. pp. 1730–17504.e4. [Google Scholar]
- 3.Clinical Laboratory Standards Institute (CLSI). Defining, establishing, and verifying reference intervals in the clinical laboratory; proposed guideline, 3rd edn. C28-A3. Clinical Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania, USA.
- 4.Kish L. A procedure for objetive respondent selection within the household. Am Stat Assoc J. 1949;44(247):380–387. doi: 10.1080/01621459.1949.10483314. [DOI] [Google Scholar]
- 5.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 6.Marwaha RK, Tandon N, Garg MK, Kanwar R, Narang A, Sastry A, et al. Bone health in healthy Indian population aged 50 years and above. Osteoporos Int. 2011;22(11):2829–2836. doi: 10.1007/s00198-010-1507-8. [DOI] [PubMed] [Google Scholar]
- 7.Meena D, Khatkhatay MI, Vijaya T, Zakiya A. Changes in cytokines, biomarkers of bone turnover and hormones are associated with bone loss in postmenopausal Indian women. Int J Endocrinol Metab. 2012;10(1):399–403. doi: 10.5812/ijem.3339. [DOI] [Google Scholar]
- 8.Jada MR, Perugu B, Chalasani S, Parvathi G. Evaluation of biochemical and bone density parameters in premenopausal and postmenopausal women: Int J Biol Med Res. 2013;4(3):3441–3443. [Google Scholar]
- 9.Jorde R, Sundsfjord J, Bønaa KH. Determinants of serum calcium in men and women. The Tromsø Study. Eur J Epidemiol. 2001;17(12):1117–1123. doi: 10.1023/A:1021272831251. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee A, editor. Population based reference standards of peak bone mineral density of indian males and females-an ICMR multi-centre task force study. osteoporosis international; 2010: springer london ltd 236 grays inn rd, 6th floor, london wc1x 8hl, England. 2010.
- 11.Cirillo M, Bilancio G, Marcarelli F. Ageing and changes in phosphate transport: clinical implications. J Nephrol. 2010;23(Suppl 16):S152–S157. [PubMed] [Google Scholar]
- 12.Mithal A, Wahl DA, Bonjour J-P, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 13.Shetty S, Kapoor N, Naik D, Asha HS, Prabu S, Thomas N, et al. Osteoporosis in healthy South Indian males and the influence of life style factors and vitamin D status on bone mineral density. J Osteoporos. 2014;2014:1–5. doi: 10.1155/2014/723238. [DOI] [PMC free article] [PubMed] [Google Scholar]


