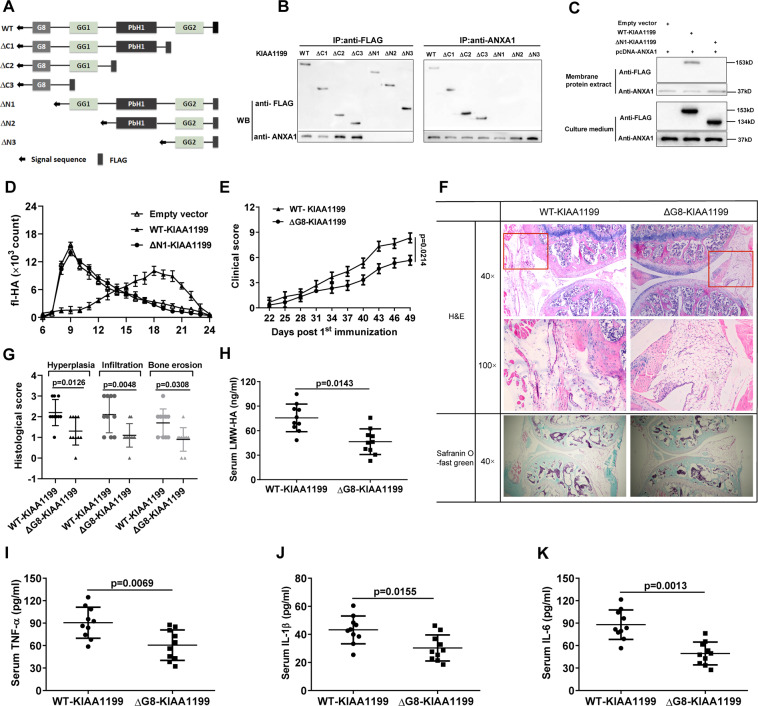
Fig. 5. G8 domain is necessary for sKIAA1199 binding to membrane ANXA1 and its in vivo function.
A Schematic diagram of KIAA1199 domain mapping. Wild-type (WT) KIAA1199 and various mutants have a N-terminal signal peptide sequence and a C-terminal FLAG tag. B The interaction of ANXA1 with WT- or various domain-deleted KIAA1199 mutants was analyzed by Co-IP assays using anti-FLAG and anti-ANXA1 antibodies. WT-KIAA1199 is a 153 kDa protein and the molecular weights of ΔC1, ΔC2, ΔC3, ΔN1, ΔN2, and ΔN3 mutants are predicted to be 95, 33, 19, 134, 105, and 40 kDa, respectively. C Detection of WT-KIAA1199 and ΔN1-KIAA1199 mutant (without G8 domain) in membrane protein extracts and in the medium of ANXA1/293T cells transfected with vectors containing WT-KIAA1199 or ΔN1-KIAA1199 cDNAs, respectively. Empty vector was used as a negative control. D The effect of secreted WT-KIAA1199 and ΔN1-KIAA1199 on HA degradation in the media mixed with the membrane fractions of ANXA1/293T cells. E Clinical scores of CIA mice with kiaa1199-KO background following injection of adenovirus-coated WT-KIAA1199 and ΔG8-KIAA1199 vectors for 4 weeks, respectively. F, G Knee joints of CIA mice were stained by H&E and safranin O-fast green (H). Histological scores for synovial hyperplasia, inflammatory cell infiltration, and cartilage and bone erosion were shown in I. H–K Serum levels of LMW-HA (H) and cytokines TNF-α (I), IL-1β (J), and IL-6 (K) in CIA mice after 4 weeks of treatment. All experiments were performed at least in triplicates, the data are presented as mean ± SD.