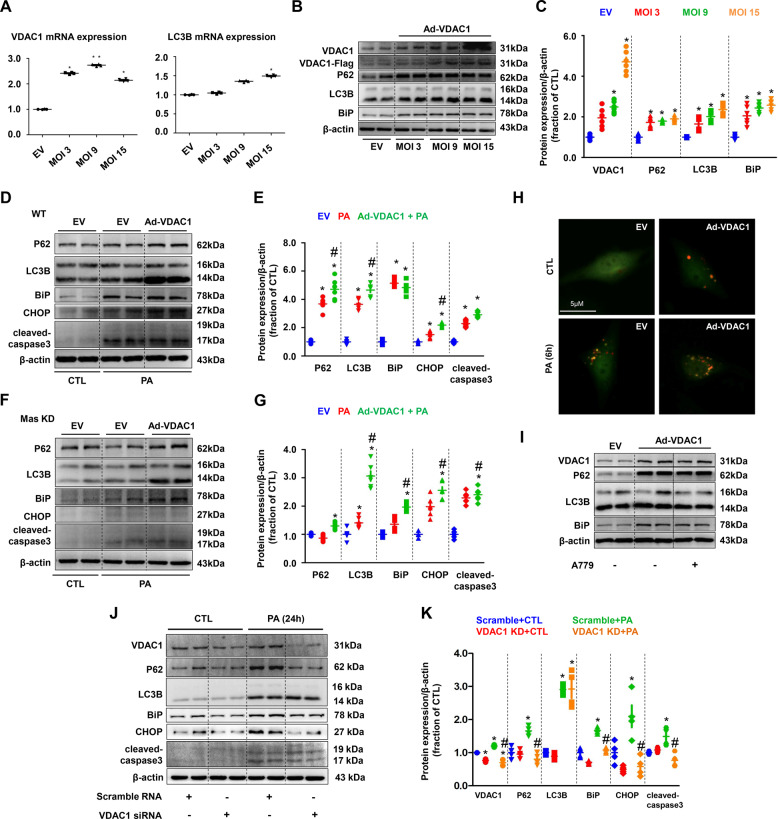
Fig. 8. VDAC1 was involved in PA-induced impaired autophagy, ER stress and cell death in HK2 cells.
A mRNA levels of VDAC1 and LC3B were examined by quantitative RT-PCR in empty vector (EV) and adenovirus encoding VDAC1 (Ad-VDAC1) transfected HK2 cells. B and C Representative immunoblots and corresponding densitometry analysis of VDAC1, P62, LC3B, and BiP protein abundance in HK2 cells transfected with Ad-VDAC1. β-actin was used as a loading control. D and G Representative immunoblots and corresponding densitometry analysis of protein markers of autophagy, ER stress and apoptosis in PA-treated WT and Mas knockdown (KD) HK2 cells transfected with Ad-VDAC1. β-actin was used as a loading control. H Confocal microscopy images of LC3B puncta formation in Ad-VDAC1 transfected HK2 cells treated with PA (400 μM) for 6 h. I Representative immunoblots and corresponding densitometry analysis of VDAC1, P62, LC3B, and BiP protein abundance in EV or Ad-VDAC1 transfected HK2 cells treated with A779. J and K Representative immunoblots and corresponding densitometry analysis of VDAC1, P62, LC3B, BiP, CHOP and cleaved-caspase-3 protein abundance in PA-treated HK2 cells transfected with VDAC1 siRNA. β-actin was used as a loading control. EV, empty vector; Ad, adenovirus. Data are shown as mean ± SEM; *P < 0.05 compared with EV + CTL; #P < 0.05 compared with EV+PA. The experiment was repeated three times.