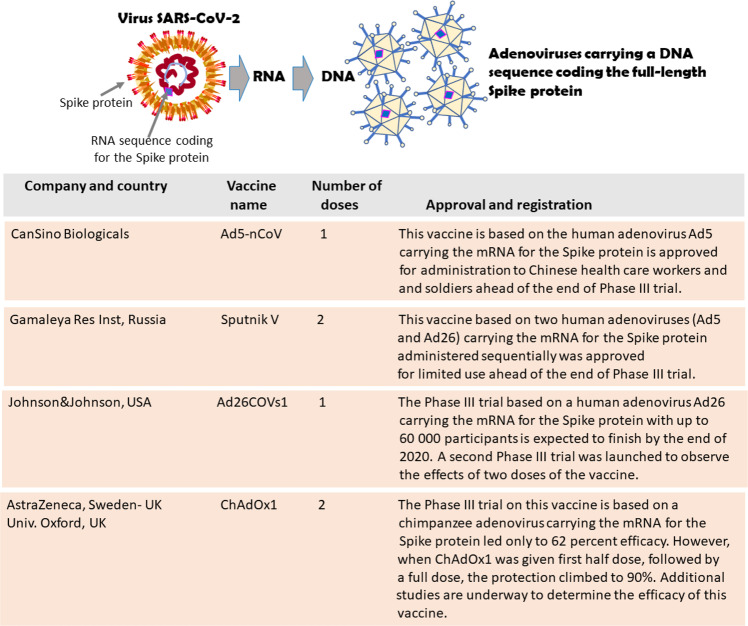
Fig. 5. Twelve candidate vaccines currently in Phase III trial. COVID-19 Vaccines based on Spike protein DNA carried by adenoviruses.
The Chinese Can Sino Ad5-nCoV and the Russian Gamaley Res. Inst. Sputnik V vaccines have already obtained a limited authorization and have been administered to sections of the population. Despite the excellent preliminary results and well-documented immunogenicity, the Phase III trial on AstraZeneca/University of Oxford ChAdOx1 vaccine provided provocative but somewhat contradictory results that require further study [8].