Figure 2.
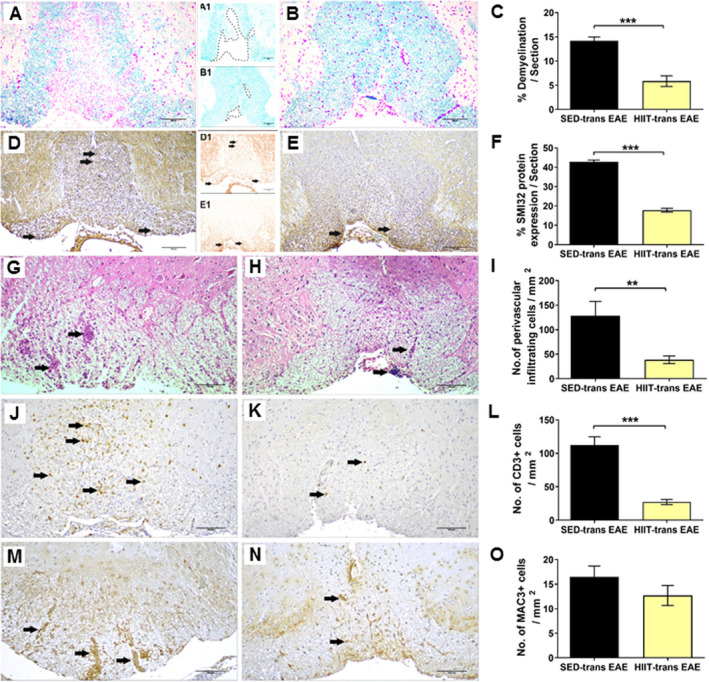
Attenuation of acute pathology in the spinal cords of experimental autoimmune encephalomyelitis (EAE) mice injected with proteolipid (PLP)‐reactive lymph node (LN) ‐T cells from high‐intensity interval trained mice. Evaluation of demyelination (A‐C), axonal damage (D‐F), and inflammation (G‐O) on cross sections of the spinal cords in EAE mice that were injected with LN‐ T cells from control sedentary (SED‐ transfer [trans] EAE; A, A1, D, D1, G, J, M; n = 6) or from high‐intensity interval trained mice (HIIT‐ trans EAE; B, B1, E, E1, H, K, N; n = 6) mice, at 12 days post EAE induction. C, F – quantification of tissue pathology in spinal cord white matter. I ‐ O – counts of inflammatory cell types in spinal cord white matter. A, B – luxol fast blue (LFB) histochemistry with periodic acid schiff (PAS) counterstaining, A1, B1 – LFB histochemistry without PAS counterstaining, dashed lines represent areas of demyelination shown in A and B, respectively; D, E – SMI32 immunohistochemistry with hematoxylin counterstaining, D1, E1 ‐ SMI32 immunohistochemistry without hematoxylin counterstaining, in brown SMI32+ injured axons. LFB staining showed reduction in the area of demyelination in HIIT‐transfer EAE (B, B1) versus SED‐transfer EAE mice (A, A1, C). SMI32 immunostainnings showed less axonal damage (F) in HIIT‐transfer EAE (E, E1) than in control SED‐transfer EAE mice (D, D1). In HIIT trans‐ EAE mice there was a reduction in total perivascular immune cell infiltrations (H), in CD3+ T cells (K) and non‐significant reduction in Mac3+ macrophages (N) versus SED tans‐EAE mice (G, J, M, respectively). G, H: arrows – indicate perivascular infiltrates; J, K: arrows – indicate perivascular CD3 + T cells; M, N: arrows – indicate Mac3 + macrophages. Scale bars = 100 μm. Data are mean ± SE. *P < 0.05, ***P < 0.001.
