Abstract
Background:
Continuous-flow (CF) left ventricular assist devices (LVADs) improve outcomes for patients with advanced heart failure (HF). However, the lack of a physiologic pulse predisposes to side-effects including uncontrolled blood pressure (BP) and there are little data regarding the impact of CF-LVADs on BP regulation.
Methods:
Twelve patients (10 males, 60±11years) with advanced HF completed hemodynamic assessment 2.7±4.1 months before, and 4.3±1.3 months following CF-LVAD implantation. Heart rate (HR) and systolic BP (SBP) via arterial catheterization were monitored during Valsalva maneuver, spontaneous breathing and a 0.05Hz repetitive squat-stand maneuver to characterize cardiac baroreceptor sensitivity. Plasma norepinephrine levels were assessed during head-up tilt (HUT) at supine, 30° and 60°. HR and BP were monitored during cardiopulmonary exercise testing (CPET).
Results:
Cardiac baroreceptor sensitivity, determined by Valsalva as well as Fourier transformation and transfer function gain of HR and SBP during spontaneous breathing and squat-stand maneuver, was impaired prior to and following LVAD implantation. Norepinephrine (NE) levels were markedly elevated pre-LVAD and improved – but remained elevated post-LVAD (supine NE pre- v. post-LVAD: 654±437 v. 323±164pg/mL). BP increased during CPET testing post-LVAD, but the magnitude of change was modest and comparable to the changes observed during the pre-LVAD CPET.
Conclusions:
Among patients with advanced HFrEF, CF-LVAD implantation is associated with modest improvements in autonomic tone, but persistent reductions in cardiac baroreceptor sensitivity. Exercise-induced increases in BP are blunted. These findings shed new light on mechanisms for adverse events such as stroke, and persistent reductions in functional capacity, among patients supported by CF-LVADs.
Keywords: Mean arterial pressure, Hypertension, Heart Failure with Reduced Ejection Fraction, Mechanical Circulatory Support, Baroreceptor Sensitivity
INTRODUCTION
Patients with advanced heart failure with reduced ejection fraction (HFrEF) suffer from severe reductions in quality-of-life, functional capacity and survival.1–4 Heart transplant remains the gold standard definitive treatment modality, but continuous-flow (CF) left ventricular assist devices (LVADs) have emerged as an attractive alternative as a bridge-to-transplantation due a supply-demand mismatch of available organs, or as destination therapy for transplant-ineligible patients. Despite improvements in survival5–7, CF-LVADs are associated with complications resulting, in part, from chronic exposure to a non-physiologic arterial pressure waveform characterized by reductions in pulsatility.8–10
Blood pressure (BP) regulation results from multiple physiologic pathways, including the sympathoadrenal and renin-angiotensin axes, as well as the arterial baroreceptor reflex pathway. Animal models11, 12 and human studies13, 14 have demonstrated that baroreceptors respond to rhythmic pulsatile distension, such as occurs during the normal cardiac cycle, to determine sympathetic nerve activity (SNA) throughout the body on a beat-to-beat basis. It is well known that autonomic tone is increased in HFrEF and that the degree of elevation is inversely associated with overall survival.15 Despite normalization of a resting cardiac output (Qc) following implantation of a CF-LVAD, these patients have increased levels of SNA, likely resulting from two unique properties of arterial perfusion associated with mechanical circulatory support: first, the lack of a physiologic pulse; and second, the body’s inability to directly regulate flow through a denervated machine with no biofeedback loop.13, 16–18
Given these unique aspects of circulatory support, it is unclear how resting and exertional BP are regulated in HFrEF patients supported by CF-LVADs. Therefore, the primary objective of this study was to provide a comprehensive assessment of arterial perfusion pressure — including cardiac baroreceptor sensitivity and changes in the BP profile in response to changes in loading conditions as well as exercise, in advanced HFrEF patients prior to, and following CF-LVAD implantation. Based on previous observations of resting and exertional hemodynamic19 and autonomic13 tone among CF-LVAD patients, we hypothesized that that there would be no appreciable change in these metrics following CF-LVAD implantation.
METHODS
The authors declare that all supporting data are available within the article.
Patient Population
Patients considered for enrollment were adults ≥18 years, with advanced HFrEF and were scheduled to undergo CF-LVAD implantation. Exclusion criteria included: 1) patients with an Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile 1-2 or who were on temporary mechanical circulatory support (e.g. Intraaortic balloon pump, Impella, or venoarterial extracorporeal membrane oxygenation) prior to CF-LVAD implantation; 2) patients who were pacemaker-dependent; 3) uncontrolled atrial or ventricular arrhythmias; 4) aortic insufficiency requiring surgical valve closure at the time of CF-LVAD implantation; 5) any non-cardiac issues that would adversely affect the ability to exercise (e.g. moderate-severe osteoarthritis, peripheral vascular disease, pulmonary disease). Because the protocol described below involved an invasive procedure (placement of an arterial catheter for BP monitoring), patients were required to have an international normalized ratio of 2.5 or less prior with testing. Prior to enrollment, the senior author (WKC) performed a detailed history and physical examination andexplained study procedures to all participants. Informed consent was obtained in accordance with guidelines as approved by the Colorado Multiple Institutional Review Board at the University of Colorado Anschutz Medical Campus. The protocol approved complies with the Declaration of Helsinki. The study was preregistered on clinicaltrials.gov (NCT03078972) and was overseen by an independent data safety and monitoring board.
Study Protocol
Two visits were completed for this study: “Visit 1” was completed prior to CF-LVAD implantation. “Visit 2” was completed following CF-LVAD implantation, after patients were discharged from index hospitalization, were fully recovered and ambulatory outpatients. The protocol for testing on Visits 1 and 2 were identical, thus allowing participants to serve as their own controls. The study was completed in a quiet, environmentally controlled laboratory with an ambient temperature of approximately 25 degrees Centigrade. Following instrumentation and setup, approximately 20 minutes of rest was allotted prior to recording of measurements for data acquisition and analysis.
Measurements
Heart rate (HR) and electrocardiography were monitored and recorded continuously by a wireless belt placed around the chest at the level of the heart (Equivital, Equivital Inc, New York, USA). Beat-by-beat HR was derived from each RR interval (RRI) identified from QRS complexes from continuous electrocardiogram. Blood pressure was monitored and continuously recorded via arterial catheterization of the radial artery, with transducers zeroed and placed at the level of the heart Breath-by-breath gas exchange parameters were monitored during the exercise protocol by indirect calorimetry (Vyntus, Vyaire Medical, Mettawa IL, USA) to oxygen uptake (VO2).
Head-up tilt and Valsalva:
Participants were placed in a supine resting position on a tilt table. Measurements were continuously recorded over 5-minutes of spontaneous breathing to obtain supine resting data. Next, participants were passively tilted to 30° and 60° for three minutes in each position. A belt was placed around the participant’s chest and legs to support his/her body weight. At each position, one minute was allowed for re-equilibration and data were recorded during the final two minutes to obtain HR, and BP. A blood sample was obtained at the end of data recording at each position to obtain plasma norepinephrine levels.
Following completion of the tilt, participants were returned to a supine position and a period of recovery was allowed. Then, a Valsalva maneuver was completed at 40mmHg for 20-seconds after a normal inspiration. Cardiac baroreceptor sensitivity was calculated by linear regression of the change in HR in response to decreases and increases in BP during phases IIa and IV of the Valsalva maneuver, respectively.20–22
Squat-stand maneuver:
Participants were placed in an upright standing position to prepare for a squat-stand maneuver. The squat-stand maneuver was performed to assess baroreflex sensitivity in response to large oscillations in BP using a physiology stimulus similar to daily life.23 Following hemodynamic stabilization, participants performed a repetitive squat-stand maneuver at a frequency of 0.05 Hz (10-second squat, followed by a 10-second stand) for 5 minutes. Participants were instructed to breathe normally to avoid performing a Valsalva maneuver during the procedure. Breathing patterns were continuously monitored via capnography. The arm into which the radial arterial catheter was inserted was supported with a Velcro sling, with the upper extremity securely positioned to ensure stability of BP recordings during the squat-stand maneuver. Following completion of the squat-stand, participants were allotted a several-minute break to allow for recovery.
Cardiopulmonary exercise test:
Participants were positioned on an upright cycle ergometer (Monark 828E, Cykelfabriken Monark AB, Sweden) to complete a symptom-limited exercise test. Workload on the ergometer was initiated at zero Watts and resistance was increased in ~6 to 12.5 Watt increments every two minutes until exhaustion.
Data Analysis
All hemodynamic data were recorded continuously in real-time by a commercially available bioinformatics platform (Powerlab, AD Instruments, Colorado Springs CO, USA) at a sampling frequency of 250 Hz. Steady-state hemodynamic values during supine rest were obtained by averaging beat-by-beat data over the 5-minute period of data collection.
For both the spontaneous data collection and repeated squat-stand maneuvers, spectral power of the RRI and systolic BP (SBP) from arterial catheter were estimated by Fourier transformation to determine RRI and SBP variability in different frequency ranges. Transfer function analysis between changes in SBP and RRI was completed to characterize baroreceptor sensitivity using methods previously described.24–29 For spontaneous breathing, spectral power of RRI, SBP, and mean values of gain, phase and coherence were calculated in the very low frequency (VLF) range, from 0.078 to 0.05Hz, the low frequency (LF) range, from 0.05-0.15Hz, and the high frequency (HF) range, from 0.15-0.35Hz.23 For the squat-stand maneuver, these parameters were analyzed at 0.05Hz, since this was the selected frequency of squatting and standing. The decision to use a frequency of 0.05Hz for the squat-stand maneuver was based on several factors. First, this frequency is well below the respiratory rate, meaning that breathing would not have a significant effect on observed oscillations in RRI or SBP. Second, the baroreflex-mediated resonance frequencies are greatest near this frequency range; thus, autonomically-mediated oscillations in RRI and SBP are likely to be enhanced at this frequency.30, 31
For cardiac baroreceptor sensitivity, transfer function gain quantifies the magnitude of the relationship between changes in SBP and RRI, where a lower gain is indicative of reduced baroreceptor sensitivity. Phase represents the temporal relationship between changes in SBP and RRI. Coherence approaching unity signifies a linear relationship between input and output signals, whereas coherence approximating zero indicates a non-linear relationship or no relationship at all between signals.
Statistical Analysis
Results are presented as mean ± standard deviation for continuous variables and frequency (%) for categorical variables. Statistical comparisons were made using linear mixed models with random effects. The models included two factors for analysis of power spectral and transfer function data (e.g., frequency and time, meaning pre- v. post-LVAD) as well as for passive tilt (e.g. tilt and time) and exercise hemodynamic data (e.g., exercise stage and time). In the case of a significant F-test, Tukey’s post-hoc testing was used for multiple comparisons. Non-normally distributed data are presented as median (interquartile range) and were analyzed using Wilcoxon sin-rank test for paired non-normally distributed data. Statistical significance was established using a P value of <0.05. Statistical analysis was performed using SAS v. 9.4 (SAS Institute Inc, Cary, NC, USA).
RESULTS
Participant Characteristics
Twelve patients with advanced HFrEF were evaluated. Visit 1 (pre-LVAD testing) and Visit 2 (post-LVAD testing) occurred at a median of 0.3 months (interquartile range: 4.3) prior to, and 4.3 (interquartile range: 2.1) months following LVAD implantation, respectively. The majority of patients were male, there was a slight predominance of nonischemic cardiomyopathy as disease etiology, and all were INTERMACS profile 3-4. Four patients were on inotropes during the pre-LVAD assessment. Of these four individuals, one patient was receiving milrinone 0.125mcg/kg/min and the other three were receiving dobutamine at an average dose of 3.2±1.6 mcg/kg/min. For all analyses that follow, the presence/absence of inotropes had no significant impact on results. As such, all analyses below include the entire cohort. Baseline demographics and medication use are displayed in Table 1.
Table 1:
Baseline characteristics
| Pre-LVAD | Post-LVAD | |
|---|---|---|
| Number | 12 | |
| Age, years | 60±11 | |
| Male sex, N (%) | 10 (83) | |
| Height, cm | 172±11 | |
| Weight, kg | 91±26 | 95±24 |
| Body mass index, kg/m2 | 30±8 | 32±6 |
| Body surface area, m2 | 2.1±0.3 | 2.1±0.3 |
| Heart Failure Etiology | ||
| Ischemic, N (%) | 5 (42) | |
| Nonischemic, N (%) | 7 (58) | |
| INTERMACS Profile | ||
| Profile 3, N (%) | 4 (33) | |
| Profile 4, N (%) | 8 (67) | |
| LVAD Type | ||
| Heartmate III, N (%) | 5 (42) | |
| Heartware VAD, N (%) | 7 (58) | |
| Medications | ||
| Beta-blocker, N (%) | 4 (33) | 2 (17) |
| ACE/ARB/ARNI, N (%) | 6 (50) | 10 (83) |
| MRA, N (%) | 11 (92) | 7 (58) |
| Hydralazine, N (%) | 4 (33) | 1 (8) |
| Isosorbide, N (%) | 4 (33) | 0 |
| Digoxin, N (%) | 8 (67) | 0 |
| Diuretic, N (%) | 11 (92) | 2 (17) |
| Inotrope, N (%) | 4 (33) | 0 |
| Hemodynamics | ||
| Heart rate, bpm | 92±18 | 88±11 |
| Systolic BP, mmHg | 117±11 | 108±23 |
| Diastolic BP, mmHg | 66±22 | 78±10 |
| Pulse pressure, mmHg | 51±28 | 30±21 |
| Mean arterial pressure, mmHg | 78±10 | 88±12 |
Hemodynamic and Autonomic Responses to Passive Tilt
HR during supine rest was similar prior to and following LVAD implantation, and there was minimal change in HR during the tilt. No patients were pacemaker-dependent. Following LVAD implantation, SBP was significantly lower and diastolic BP (DBP) was higher (table 1 and figure 1). Consequently, pulse pressure (PP) and mean arterial pressure (MAP) were significantly decreased and increased, respectively, As demonstrated in figure 1, there was a slight, non-significant reduction in MAP during tilt, but the degree of change experienced by participants was similar prior to and following LVAD implantation. Prior to LVAD implantation, plasma norepinephrine levels were severely abnormal and progressively increased throughout the tilt. Post-LVAD, supine resting norepinephrine levels were significantly lower than pre-implantation levels, and increased modestly during the tilt. The magnitude of the increase in norepinephrine levels was similar to changes observed prior to LVAD implantation.
Figure 1:
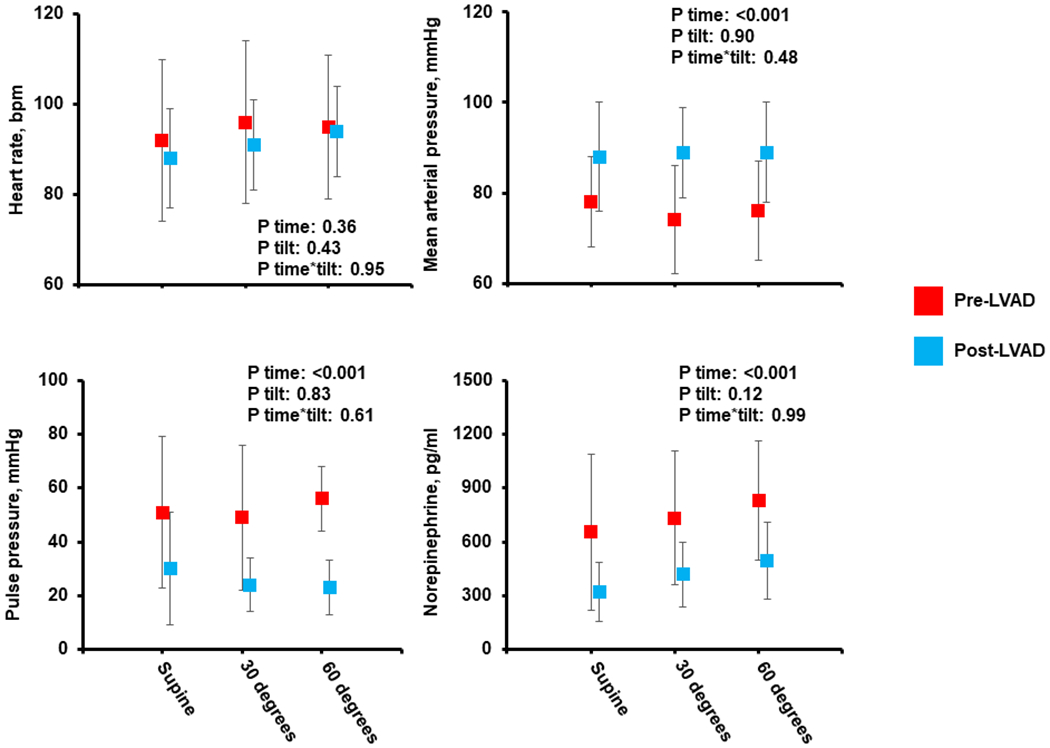
Hemodynamic and Autonomic Response to Passive Tilt
Assessment of Baroreceptor Sensitivity in the Setting of Mechanical Circulatory Support
During the Valsalva maneuver (figure 2), changes in RRI in response to reductions in BP during phase IIa were similar among patients prior to and following LVAD implantation (pre v. post-LVAD: 0.34±0.99ms/mmHg v. 0.42±1.08ms/mmHg, P=0.89). RRI responses during phase IV of the Valsalva were also similar before and after CF-LVAD implantation (pre v. post-LVAD: 0.47±1.74ms/mmHg v. 0.38±0.29ms/mmHg, P=0.90).
Figure 2:
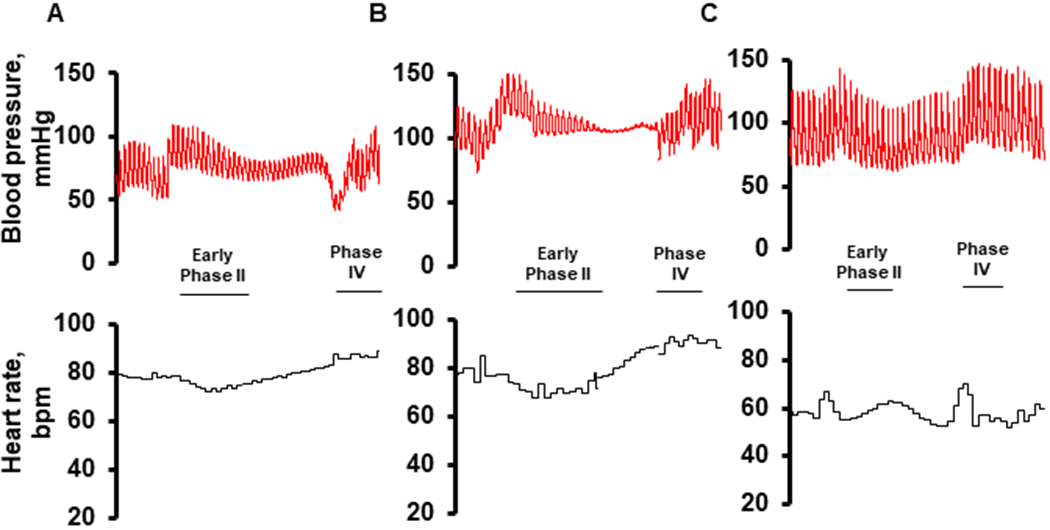
Hemodynamic Response to Valsalva Maneuver. Example tracings taken during a Valsalva maneuver from a single participant prior to (A), and following (B) LVAD implantation. Note the marked reduction in pulsatility throughout phase II of the Valsalva maneuver. For a referent comparison of a normal response, Valsalva maneuver obtained from a healthy control is included (C).
Fourier transformation of data obtained during spontaneous breathing demonstrated that RRI and SBP variability were similar prior to, and following LVAD implantation in all frequency ranges (table 2). Transfer function gain in the VLF and LF ranges were similar prior to and following LVAD implantation, indicating there was no change in cardiac baroreceptor sensitivity. Nine participants completed the 0.05Hz (10-second) squat-stand maneuver – three were unable to complete the test due to leg fatigue. Power spectral data at 0.05Hz for both RRI and SBP were similar prior to and following device implantation (RRI power spectral data pre v. post-LVAD: 756±845 v. 559±454ms2 Hz−1, P=0.62; SBP power spectral data pre v. post-LVAD: 2806±2303 v. 5767±4182mmHg2 Hz−1, P=0.18). At 0.05Hz, transfer function gain of RRI and SBP were similar prior to and following LVAD implantation (Gain pre- v. post-LVAD: 0.50±0.31 v. 0.35±0.23ms mmHg−1, P=0.53; Coherence pre- v. post-LVAD: 0.72±0.17 v. 0.77±0.14 units, P=.60).
Table 2:
Power Spectral and Transfer Function Analysis of R-R Interval and Systolic Blood Pressure During Spontaneous Breathing
| Pre-LVAD | LVAD | Change | P-value | |
|---|---|---|---|---|
| Very Low Frequency (0.078-0.05Hz) | ||||
| Power Spectral Analysis | ||||
| PS-RRI, ms2 Hz−1 | 1239 (591, 2126) | 1723 (613, 3098) | 704 (−953, 1828) | 0.59 |
| PS-SBP, mmHg2 Hz−1 | 68 (13, 104) | 76 (13, 111) | −10 (−26, 13) | 0.47 |
| Transfer Function Analysis | ||||
| Gain, ms mmHg−1 | 1.8 (1.1, 2.7) | 2.2 (1.3, 3.8) | 1.0 (−1.0, 1.8) | 0.93 |
| Phase, radians | −0.23 (−1.01, 0.59) | −0.24 (−1.03, 0.28) | −0.04 (−1.40, 0.86) | 0.38 |
| Coherence, units | 0.40 (0.32, 0.52) | 0.43 (0.37, 0.55) | −0.11 (−3.74, 0.18) | 0.89 |
| Low Frequency (0.05-0.15Hz) | ||||
| Power Spectral Analysis | ||||
| PS-RRI, ms2 Hz−1 | 155 (55, 451) | 426 (145, 839) | 151 (−6, 693) | 0.26 |
| PS-SBP, mmHg2 Hz−1 | 16 (3, 48) | 31 (3, 53) | 13 (−17, 39) | 0.60 |
| Transfer Function Analysis | ||||
| Gain, ms mmHg−1 | 1.8 (1.2, 3.4) | 2.4 (0.6, 2.8) | 0.2 (−1.5, 1.3) | 0.73 |
| Phase, radians | −0.79 (−1.20, −0.18) | −0.21 (−0.83, 0.16) | 0.34 (−1.11, 1.04) | 0.13 |
| Coherence, units | 0.37 (0.34, 0.54) | 0.36 (0.29, 0.48) | −0.16 (−0.23, 0.13) | 0.73 |
| High Frequency (0.15-0.35Hz) | ||||
| Power Spectral Analysis | ||||
| PS-RRI, ms2 Hz−1 | 70 (33, 329) | 196 (71, 456) | 5 (−98, 205) | 0.42 |
| PS-SBP, mmHg2 Hz−1 | 6 (4, 28) | 28 (4, 55) | 24 (−5, 39) | 0.33 |
| Transfer Function Analysis | ||||
| Gain, ms mmHg−1 | 1.5 (0.9, 3.1) | 1.6 (0.9, 2.3) | −0.1 (−0.8, 1.4) | 0.65 |
| Phase, radians | −0.69 (−0.88, −0.18) | −0.15 (−0.61, 0.44) | 0.56 (−0.43, 1.11) | 0.13 |
| Coherence, units | 0.37 (0.30, 0.56) | 0.37 (0.35, 0.44) | −0.02 (−0.39, 0.70) | 0.95 |
Data presented as median with interquartile range. “Change” represents the difference in values between data obtained prior to, and following LVAD implantation. PS: power spectral; RRI: RR interval; SBP: systolic blood pressure.
Heart Rate and Blood Pressure Response During Exercise
Peak workloads obtained during upright cycle ergometry were similar prior to and following LVAD implantation (pre v. post 20±21 v. 31±18 Watts, P=0.16). VO2Max also did not improve (pre v. post: 0.95±0.31 v. 1.02±0.32L/min, P=0.28; 10.5±2.7 v. 11.0±3.3ml/kg/min, P=0.44). Prior to LVAD implantation, HR increased significantly from rest to peak exercise and peak percent HR achieved was 78±13%. MAP increased modestly from 79±12 to 92±16mmHg.
Following LVAD implantation, peak HR increased significantly from rest to peak exercise and peak percent HR achieved was 83±10%. Resting pulse pressure was significantly reduced compared to pre-LVAD levels, and increased significantly during exercise from 28±14 to 59±26mmHg. Resting MAP was significantly higher than pre-LVAD levels and increased significantly from rest to peak exercise. The overall HR and BP response to exercise is displayed in table 3. An example tracing of HR and BP responses during exercise from a single participant is displayed in figure 3.
Table 3:
Exercise Hemodynamics
| Pre-LVAD | LVAD | P-value Time | P-value Stage | P-value Time*Stage | |
|---|---|---|---|---|---|
| Heart Rate, bpm | 0.13 | <0.001 | 0.70 | ||
| Rest | 88±18 | 92±12 | |||
| Peak | 126±22* | 133±16* | |||
| Systolic BP, mmHg | 0.94 | 0.001 | 0.48 | ||
| Rest | 115±19 | 111±19 | |||
| Peak | 136±28 | 141±27* | |||
| Diastolic BP, mmHg | <0.001 | 0.16 | 0.047 | ||
| Rest | 61±10 | 84±12† | |||
| Peak | 70±12 | 82±12† | |||
| Pulse Pressure, mmHg | 0.005 | <0.001 | 0.08 | ||
| Rest | 54±13 | 28±14† | |||
| Peak | 66±21 | 59±26* | |||
| MAP, mmHg | 0.002 | 0.004 | 0.53 | ||
| Rest | 79±12 | 93±13† | |||
| Peak | 92±16 | 101±14 | |||
P<0.05 compared to rest within same group
P<0.05 compared to Pre-LVAD value at same exercise stage
Figure 3.
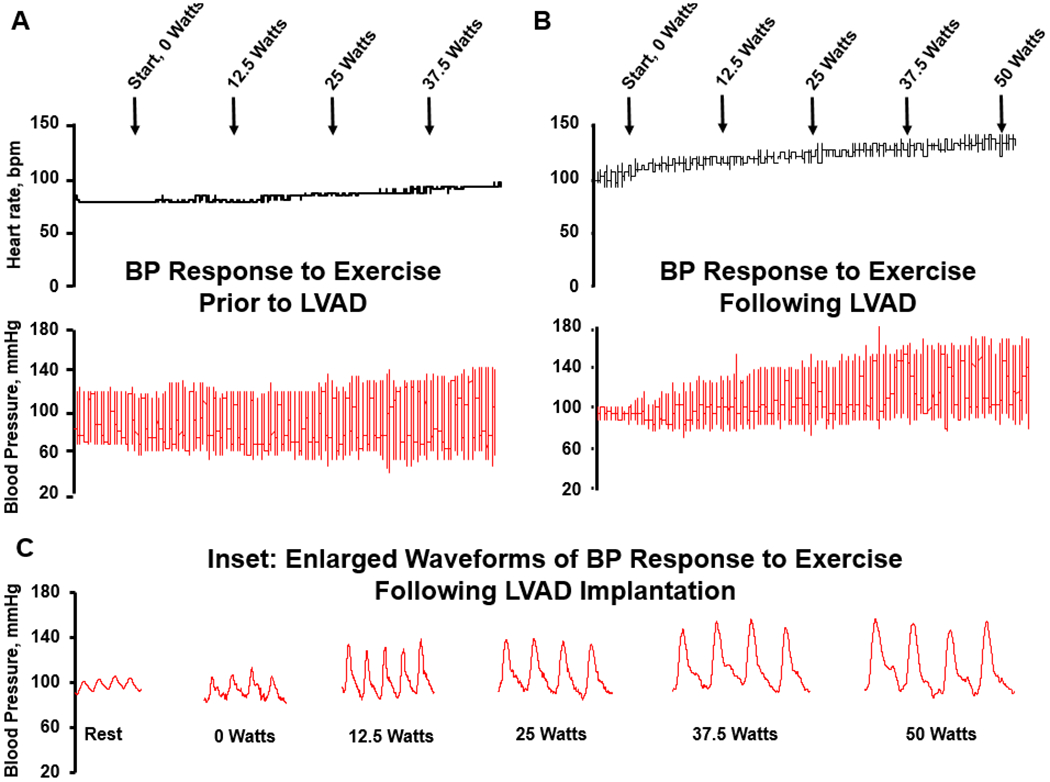
Example tracing of the heart rate and blood pressure response to exercise in a 46yo man with history of advanced HFrEF. (A): exercise prior to LVAD implantation; (B): exercise test following LVAD implantation; (C): inset demonstrates enlarged BP waveforms from B, emphasizing the early increase in pulsatility following initiation of exercise. Note dicrotic notch in arterial waveform, demonstrating aortic valve opening.
DISCUSSION
The primary findings from this study are as follows. Among patients with advanced HFrEF, 1) Cardiac baroreceptor sensitivity is reduced and does not improve following LVAD implantation. Oscillations in HR and BP, both during resting conditions and in response to provocative maneuvers that alter loading conditions, are similar prior to and following CF-LVAD implantation; 2) Autonomic tone, as determined by plasma norepinephrine concentration, is markedly abnormal in supine and upright positions. Following CF-LVAD implantation, autonomic tone is modestly reduced but remains abnormal; and 3) BP increases modestly during exercise but the overall BP response is blunted. Following CF-LVAD implantation, resting MAP is higher than pre-implantation levels, but BP reserve remains blunted, with only modest increases observed from rest to peak exercise.
This study represents the first report of advanced HFrEF patients who underwent a comprehensive analysis of BP regulation prior to and following CF-LVAD implantation. This study design allowed for individuals to serve as their own controls, and thus directly characterize the effect of mechanical circulatory support on BP regulation in humans, both at rest and during exercise, and in response to provocative challenges that alter loading conditions including a Valsalva, passive tilt, and squat-stand maneuver.
Blood Pressure Profiles in the Setting of Mechanical Circulatory Support
We found that several changes in the BP profile occurred following CF-LVAD implantation that impacts BP regulation. First, DBP increased compared to pre-implantation levels, which likely results from the fact that flow through the device is delivered continuously and is not directly gated to the cardiac cycle. The rise in DBP contributes to increases in MAP and may also contribute to increased levels of DBP during exercise that occur in these patients, in contrast to normal individuals, where DBP may decline during exercise as a result of peripheral vasodilatation. Indeed, CF-LVAD patients are at increased risk of elevated BP and overt hypertension, and frequently require multiple classes of anti-hypertensive medications to reduce the risk of adverse events.16, 17, 32, 33
Not surprisingly, we also observed a significant reduction in pulsatility following CF-LVAD implantation. However, there was a high degree of variability in pulse pressure between individuals, with some having minimal pulsatility and others having a near-physiologic pulse. There are several factors that may account for this pulsatility even during supine resting conditions. First, axial-flow and centrifugal-flow pumps are preload34 and afterload35 sensitive devices. Centrifugal-flow devices have a flatter H-Q curve than their axial-flow counterparts, meaning these devices theoretically provide higher levels of pulsatility in response to changes in pressure differential across the pump.35 Thus, while CF-LVAD flow is not gated directly to the cardiac cycle, pulsatile delivery of flow may occur as the pressure differential across the pump changes throughout the cardiac cycle. Second, automated modulations in pump speed, such as occurs in the Heartmate 3 at a frequency of 0.5Hz (every other second), and the Heartware VAD, which incorporates the Lavare cycle once per minute, wash pump bearings to reduce the rate of thrombus formation and it is possible that these pump speed modulations impart a modicum of pulsatility systemically. Finally, pulsatility may result from intrinsic contractility of the native ventricle. Over time, ventricular unloading may allow for varying degrees of recovery/improvement in ventricular function, meaning that pulsatility may increase over time.
Cardiac Baroreceptor Sensitivity and Autonomic Tone
It is well established that advanced HFrEF is associated with a hyperadrenergic environment characterized by markedly elevated levels of circulating catecholamines and SNA, along with reduced sympathetic and vagal HR modulation, and impaired sympathetic regulation of peripheral resistance.15, 36–38 Furthermore, baroreflex-mediated HR responses to BP changes are diminished and are associated with decrements in survival.39–42 However, following CF-LVAD implantation, general knowledge of baroreceptor responsiveness as well as its impact on BP control/regulation, and whether there is improvement in baroreceptor-mediated changes in HR in response to alterations in BP, is unknown.16
We analyzed longitudinal changes in cardiac baroreceptor sensitivity through three methods – Fourier transformation and transfer function analysis of oscillations in HR and SBP during spontaneous breathing, during a 0.05Hz squat-stand maneuver, and a Valsalva maneuver – all of which are well validated methods of assessing cardiac baroreceptor sensitivity.21–23 By all three methods, we found that there is no meaningful change in baroreceptor sensitivity following CF-LVAD implantation and that overall, cardiac baroreceptor sensitivity is reduced. This observation is demonstrated by visualization of the hemodynamic response to Valsalva maneuver. The normal Valsalva response (Figure 2C) involves noticeable increases in HR during phase IIa as SBP declines, and noticeable reductions in HR during the hypertensive overshoot (phase IV). In our patients, group-averaged data demonstrate that HR does change during these phases of the Valsalva, but the magnitude of the change is diminished compared to normal.22 For example, in normal individuals, cardiac baroreceptor sensitivity during the Valsalva maneuver is on the order of ~5-8ms/mmHg during phase IIa and ~6-15ms/mmHg during phase IV22, whereas we have observed sensitivity levels of less than 1ms/mmHg for phase IIA and phase IV in the LVAD patients.
Not surprisingly, the level of circulating norepinephrine levels was markedly elevated among patients with advanced HFrEF.43 During tilt, sympathetic nerve traffic increases to maintain peripheral resistance – and overall peripheral perfusion, as Qc decreases. Not surprisingly, we observed large increases in norepinephrine as HFrEF patients progressed through the tilt. Following CF-LVAD implantation, we found that supine resting norepinephrine levels were reduced at 323±164pg/ml, but still above levels expected among healthy persons at similar age (approximately 200-250pg/ml for a healthy 60-70 year-old).44–46 These findings complement previous studies that have reported elevated levels of SNA among CF-LVAD patients, though those studies were limited insomuch as they involved an assessment at a single point in time, namely after CF-LVAD implantation.13, 14 Our data add to this body of literature by demonstrating that while sympathetic tone (as measured by norepinephrine levels) is elevated following CF-LVAD implantation, there is a modest improvement compared to levels observed in the setting of advanced HFrEF prior to CF-LVAD implantation. This improvement likely results from normalization of a resting Qc, which reduces the body’s need to increase peripheral resistance to maintain arterial perfusion.
Blood Pressure Response to Exercise
Following CF-LVAD implantation, we found that MAP increased significantly from rest to peak exercise. Interestingly, we observed a substantial increase in pulsatility throughout the duration of the test. This rise in pulsatility was observed immediately upon initiation of exercise (figure 3, part C), as evidenced by an increase in the arterial pressure waveform and the presence of a dicrotic notch, indicative of augmentation in contractility of the native ventricle and opening of the aortic valve. That said, the overall magnitude of the rise in MAP from rest to peak exercise was modest, and comparable to the degree of changes observed during exercise testing prior to device implantation. Taken together, these observations suggest that CF-LVAD patients have a modest degree of contractile reserve, but that functionally, the BP response to exercise may be blunted.
Clinical Implications of Impaired BP Regulation Among CF-LVAD Patients
Strokes are among the most feared of all device-related complications, and between 6-24 months of support, are the most common cause of death for these patients.16, 47 The risk of stroke may be increased due to a host of issues, such as arrhythmias, pump thrombus, device-related infections and adjustments in anticoagulation.16 However, elevated BP is a major risk factor for stroke, particularly among patients supported by the Heartware VAD.16, 33 Our data demonstrate that increases in MAP appear to be driven by elevations in DBP that result from continuous-flow throughout the duration of the cardiac cycle. These findings also emphasize the importance of antihypertensive therapy in this population to minimize stroke-risk.
The second major clinical implication of our findings is in regards to exercise capacity. It is well known that CF-LVAD patients have persistent limitations in VO2Max, and while the six-minute hallwalk scores increase, the magnitude of change is modest and in many cases, the actual distance achieved falls within values that are characteristic of patients with advanced HFrEF (e.g. 300-350m).48 We have documented hemodynamic evidence that contractility increases early upon initiation of exercise. This finding, in conjunction with growing evidence that augmentations in flow through the device itself are limited during exercise49, emphasizes the central role of the native ventricle in maintaining BP during exercise. Along these lines, our data indicate that during exercise, CF-LVAD patients function similarly to patients with advanced HFrEF, which emphasizes the need for ventricular recovery of function following device implantation, either through medical therapy, recovery strategies, cardiac rehabilitation, or a combination of the three.
There are limitations to our study. First, the sample size was relatively small, which may limit generalizability. However, the unique design of our study allowed patients to serve as their own controls to directly determine the impact of a CF-LVAD on BP regulation. Further, we used a gold-standard assessment of BP – ie, radial arterial catheterization, for BP assessments, which increased the reliability and accuracy of our measurements, and allowed for characterization of BP on a beat-to-beat basis. We were not powered – nor did we intend, to make direct comparisons in hemodynamic responses to different types of CF-LVADs. Rather, our overall objective was to characterize BP behavior when arterial perfusion is dependent – at least in part, on a denervated machine with no biofeedback loop. Second, our patients were assessed 4.3±1.3 months following device implantation. Our objective was to allow patients adequate time to stabilize and fully recover from device implantation, and perform hemodynamic assessments when they were discharged, ambulatory and had resumed a normal lifestyle. We did not perform serial assessments in the post-implantation period, and we cannot rule out the possibility that BP regulation may change over time, particularly if ventricular function improves and has an increased contribution to Qc. Third, we cannot rule-out the possibility of a “training effect”, whereby initial testing prior to LVAD implantation led to test-associated sympathetic activation due anticipatory excitement, which was not present on the follow-up exercise assessment following device implantation. That said, we went to great lengths to ensure that all hemodynamic parameters were stable and that patients were comfortable and relaxed during data acquisition. Finally, we did not make any medication adjustments prior to the day of testing (ie, withholding medications) due to safety reasons. While it is possible that medical therapy may have influenced some of the HR and BP responses during testing, our data nevertheless provide a “real-world” depiction of BP among CF-LVAD patients, who are prescribed classes of medications that are similar to those used by patients in our cohort.
CONCLUSIONS
CF-LVAD implantation normalizes a resting Qc, which leads to modest improvements in – but not normalization of, autonomic tone among patients with advanced HFrEF. However, cardiac baroreceptor sensitivity does not change after device implantation and overall, remains impaired. During exercise, BP reserve is blunted and the magnitude of increase in MAP is comparable to changes observed in the setting of advanced HFrEF. These observations directly explain the impact of CF-LVAD implantation on resting and exertional BP regulation among individuals with advanced HFrEF.
What is New?
Patients with advanced heart failure with reduced ejection fraction (HFrEF) suffer from physiologic alterations in autonomic tone, baroreceptor sensitivity and blood pressure (BP) regulation. The impact of left ventricular assist devices (LVAD) on these physiologic parameters remains largely unknown.
LVADs improve, but do not normalize, the hyperadrenergic environment that characterizes advanced HFrEF. Cardiac baroreceptor sensitivity remains impaired following device implantation.
Submaximal and peak exercise are characterized by large increases in pulsatility resulting from increased ventricular contractility. However, the BP response overall is blunted, with only modest increases in mean arterial pressure from rest to peak exercise.
What are the Clinical Implications?
Despite improvements in subjective assessments of quality-of-life following LVAD implantation, objective measures of functional capacity remain severely reduced. This persistently reduced exercise tolerance is due – in large part, to the inability of LVADs to augment BP during exercise.
Strokes are among the most common and feared complications following LVAD implantation. This increased risk of stroke results from persistent impairments in cardiac baroreceptor sensitivity and only partial reduction in sympathetic tone.
These findings emphasize the importance of antihypertensive therapy to ensure that BP is adequately controlled following LVAD implantation.
Acknowledgments
Funding Sources: Dr. Cornwell is supported by an NIH/NHLBI Mentored Patient-Oriented Research Career Development Award (#1K23HLI32048-01) and the Colorado Clinical Translational Science Institute (UL1TR002535 from NCATS/NIH) at the University of Colorado Anschutz Medical Campus. Dr. Tarumi is supported by the Japan Society for Promotion of Science (19K19970).
Conflicts of Interest/Disclosures: Dr. Pal is a consultant for Medtronic Corp and has received research funding from Medtronic Corp. Dr. Cornwell is a consultant for Medtronic Corp and has received research funding from Medtronic Corp.
ABBREVIATIONS:
- BP
blood pressures
- CF-LVAD
continuous-flow left ventricular assist device
- DBP
diastolic blood pressure
- HFrEF
heart failure with preserved ejection fraction
- HR
heart rate
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- MAP
mean arterial pressure
- PP
pulse pressure
- RRI
RR interval
- SBP
systolic blood pressure
Footnotes
Clinical Trial Registration: clinicaltrials.gov identifier: NCT03078972
CITATIONS
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97. [DOI] [PubMed] [Google Scholar]
- 2.Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A and Grobbee DE. The prognosis of heart failure in the general population: The Rotterdam Study. European heart journal. 2001;22:1318–27. [DOI] [PubMed] [Google Scholar]
- 3.Ho KK, Pinsky JL, Kannel WB and Levy D. The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology 1993;22:6A–13A. [DOI] [PubMed] [Google Scholar]
- 4.Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr., and Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–70. [DOI] [PubMed] [Google Scholar]
- 5.Mehra MR, Goldstein DJ, Uriel N, Cleveland JC, Yuzefpolskaya M, Salerno C, Walsh MN, Milano CA, Patel CB, Ewald GA, et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. New England Journal of Medicine. 2018;378:1386–1395. [DOI] [PubMed] [Google Scholar]
- 6.Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38:114–126. [DOI] [PubMed] [Google Scholar]
- 7.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. The New England journal of medicine. 2017;376:451–460. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan C, Kanwar M, Cockcroft JR, McDonnell B, Stohr EJ and Cornwell WK 3rd. Bionic women and men - Part 4: Cardiovascular, cerebrovascular and exercise responses among patients supported with left ventricular assist devices. Exp Physiol. 2020;105:763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castagna F, Stohr EJ, Pinsino A, Cockcroft JR, Willey J, Reshad Garan A, Topkara VK, Colombo PC, Yuzefpolskaya M and McDonnell BJ. The Unique Blood Pressures and Pulsatility of LVAD Patients: Current Challenges and Future Opportunities. Current hypertension reports. 2017;19:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purohit SN, Cornwell WK 3rd, Pal JD, Lindenfeld J and Ambardekar AV. Living Without a Pulse: The Vascular Implications of Continuous-Flow Left Ventricular Assist Devices. Circulation Heart failure. 2018;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapleau MW, Heesch CM and Abboud FM. Prevention or attenuation of baroreceptor resetting by pulsatility during elevated pressure. Hypertension. 1987;9:III137–III137. [DOI] [PubMed] [Google Scholar]
- 12.Chapleau MW, Hajduczok G and Abboud FM. Pulsatile activation of baroreceptors causes central facilitation of baroreflex. American Journal of Physiology - Heart and Circulatory Physiology. 1989;256:H1735–H1741. [DOI] [PubMed] [Google Scholar]
- 13.Markham DW, Fu Q, Palmer MD, Drazner MH, Meyer DM, Bethea BT, Hastings JL, Fujimoto N, Shibata S and Levine BD. Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circulation Heart failure. 2013;6:293–9. [DOI] [PubMed] [Google Scholar]
- 14.Cornwell WK 3rd, Tarumi T, Stickford A, Lawley J, Roberts M, Parker R, Fitzsimmons C, Kibe J, Ayers C, Markham D, et al. Restoration of Pulsatile Flow Reduces Sympathetic Nerve Activity Among Individuals With Continuous-Flow Left Ventricular Assist Devices. Circulation. 2015;132:2316–22. [DOI] [PubMed] [Google Scholar]
- 15.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB and Rector TS. Plasma Norepinephrine as a Guide to Prognosis in Patients with Congestive Heart Failure. New England Journal of Medicine. 1984;311:819–823. [DOI] [PubMed] [Google Scholar]
- 16.Cornwell WK III, Ambardekar AV, Tran T, Pal J, Cava L, Lawley J, Tarumi T, Cornwell C and Aaronson KD. Stroke Incidence and Impact of Continuous-Flow Left Ventricular Assist Devices on Cerebrovascular Physiology. Stroke. 2019;50:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchanan C, Kanwar M, Cockroft JR, McDonnell B, Stohr EJ and Cornwell WK III. Bionic women and men part 4 - cardiovascular, cerebrovacsular and exercise responses among patients supported with left ventricular assist devices. Experimental Physiology. 2020; 105(5): 763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stohr EJ, Cornwell WK 3rd, Kanwar M, Cockcroft JR and McDonnell BJ. Bionic women and men - Part 1: Cardiovascular lessons from heart failure patients implanted with left ventricular assist devices. Exp Physiol. 2020;105:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brassard P, Jensen AS, Nordsborg N, Gustafsson F, Moller JE, Hassager C, Boesgaard S, Hansen PB, Olsen PS, Sander K, et al. Central and peripheral blood flow during exercise with a continuous-flow left ventricular assist device: constant versus increasing pump speed: a pilot study. Circulation Heart failure. 2011;4:554–60. [DOI] [PubMed] [Google Scholar]
- 20.Rostagno C, Felici M, Caciolli S, Olivo G, Comeglio M, Galanti G, Gastone G and Serneri N. Decreased baroreflex sensitivity assessed from phase IV of Valsalva maneuver in mild CHF. Angiology. 1999;50:655–664. [DOI] [PubMed] [Google Scholar]
- 21.Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K and Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy: a potential mechanism for long term morbidity? Hypertension. 2005;45:513–21. [DOI] [PubMed] [Google Scholar]
- 22.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF and Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. The Journal of physiology. 2009;587:2019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Claassen JA, Shibata S, Kilic S, Martin-Cook K, Diaz-Arrastia R and Levine BD. Arterial-cardiac baroreflex function: insights from repeated squat-stand maneuvers. American journal of physiology Regulatory, integrative and comparative physiology. 2009;297:R116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornwell WK 3rd, Tarumi T, Aengevaeren VL, Ayers C, Divanji P, Fu Q, Palmer D, Drazner MH, Meyer DM, Bethea BT, et al. Effect of pulsatile and nonpulsatile flow on cerebral perfusion in patients with left ventricular assist devices. J Heart Lung Transplant. 2014;33:1295–303. [DOI] [PubMed] [Google Scholar]
- 25.Claassen JLB, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. Journal of Applied Physiology. 2009;106:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang RZJ, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. American Journal of Physiology Heart and Circulatory Physiology. 1998;274:H233–H241. [DOI] [PubMed] [Google Scholar]
- 27.Zhang RZJ, Giller CA, Levine BD. Autonomic Neural Control of Dynamic Cerebral Autoregulation in Humans. Circulation. 2002;106:1814–1820. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki KI, Zhang R, Zuckerman JH and Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults - how much training for what benefit. Journal of Applied Physiology. 2003;95:1575–1583. [DOI] [PubMed] [Google Scholar]
- 29.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R and Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol (1985). 2005;99:1041–9. [DOI] [PubMed] [Google Scholar]
- 30.van de Vooren H, Gademan MG, Swenne CA, TenVoorde BJ, Schalij MJ and Van der Wall EE. Baroreflex sensitivity, blood pressure buffering, and resonance: what are the links? Computer simulation of healthy subjects and heart failure patients. J Appl Physiol (1985). 2007;102:1348–56. [DOI] [PubMed] [Google Scholar]
- 31.Hammer PE and JP S. Resonance in a mathematical model of baroreflex control: arterial blood pressure waves accompanying postural stress. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2005;288:R1637–R1648. [DOI] [PubMed] [Google Scholar]
- 32.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, Starling RC, Chen L, Boyle AJ, Chillcott S, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–39. [DOI] [PubMed] [Google Scholar]
- 33.Saeed O, Jermyn R, Kargoli F, Madan S, Mannem S, Gunda S, Nucci C, Farooqui S, Hassan S, McLarty A, et al. Blood pressure and adverse events during continuous flow left ventricular assist device support. Circulation Heart failure. 2015;8:551–6. [DOI] [PubMed] [Google Scholar]
- 34.Fukamachi K, Shiose A, Massiello A, Horvath DJ, Golding LA, Lee S and Starling RC. Preload sensitivity in cardiac assist devices. Ann Thorac Surg. 2013;95:373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagani FD. Continuous-flow rotary left ventricular assist devices with “3rd generation” design. Seminars in thoracic and cardiovascular surgery. 2008;20:255–63. [DOI] [PubMed] [Google Scholar]
- 36.Leimbach WN, Wallin BG, Victor RG, Aylward PE, Sundlof G and Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. [DOI] [PubMed] [Google Scholar]
- 37.Zhang DY and Anderson AS. The sympathetic nervous system and heart failure. Cardiology clinics. 2014;32:33–45, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. Journal of the American College of Cardiology. 2009;54:375–85. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson DW, Berg WJ, Roach PJ, Oren RM and Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity American Journal of Cardiology. 1992;69:523–531. [DOI] [PubMed] [Google Scholar]
- 40.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB and Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92:3206–3211. [DOI] [PubMed] [Google Scholar]
- 41.Eckberg DL, Drabinsky M and Braunwald E. Defective cardiac parasympathetic ocntrol in patients with heart disease. New England Journal of Medicine. 1971;285:877–883. [DOI] [PubMed] [Google Scholar]
- 42.La Rovere MT, Pinna GD, Maestri R, Robbi E, Caporotondi A, Guazzotti G, Sleight P and Febo O. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. Journal of the American College of Cardiology. 2009;53:193–9. [DOI] [PubMed] [Google Scholar]
- 43.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G and Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. Journal of the American College of Cardiology. 2009;54:1747–62. [DOI] [PubMed] [Google Scholar]
- 44.Fiorica V Plasma norepinephrine levels of elderly men on a controlled sodium intake diet. journal of the American Geriatrics Society. 1984;32:576–580. [DOI] [PubMed] [Google Scholar]
- 45.Veith RC, Featherstone JA, Linarest OA and Halter JB. Age Differences in Plasma Norepinephrine Kinetics in Humans. Journal of Gerontology. 1986;41:319–324. [DOI] [PubMed] [Google Scholar]
- 46.Shimada K, Kitazumi T and Sadakane N. Age-related changes of baroreflex function, plasma norepinephrine and blood pressure. hypertension. 1985;7:113–117. [DOI] [PubMed] [Google Scholar]
- 47.Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB and Naftel DC. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. [DOI] [PubMed] [Google Scholar]
- 48.Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, Edwards BS, Park S, John R, Conte JV, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. Journal of the American College of Cardiology. 2010;55:1826–34. [DOI] [PubMed] [Google Scholar]
- 49.Moss N, Rakita V, Lala A, Parikh A, Roldan J, Mitter SS, Anyanwu A, Campoli M, Burkhoff D and Mancini DM. Hemodynamic Response to Exercise in Patients Supported by Continuous Flow Left Ventricular Assist Devices. JACC Heart Fail. 2020;8:291–301. [DOI] [PubMed] [Google Scholar]


