Figure 1.
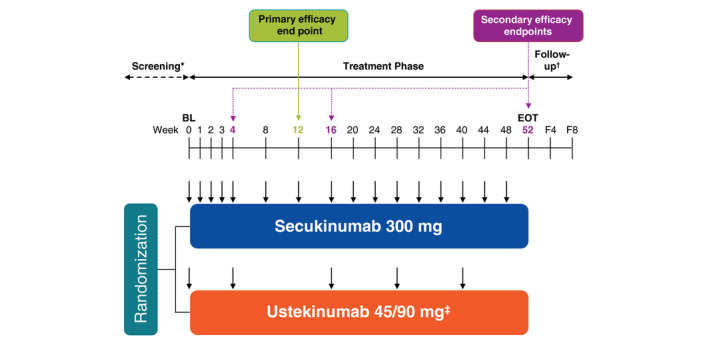
Design of the CLARITY study. BL, baseline; EOT, end of treatment phase; F4, follow‐up visit approximately 4 weeks after the EOT visit; F8, follow‐up visit approximately 8 weeks after the EOT visit. *The screening phase duration was at least 2 weeks and up to 4 weeks. †For patients with premature treatment discontinuation only. ‡Ustekinumab dose was based on bodyweight at baseline; 45 mg for patients ≤100 kg; 90 mg for patients >100 kg. ↓Active dose administration; patients received placebo at several time points to maintain blinding (not shown).
