Figure 4.
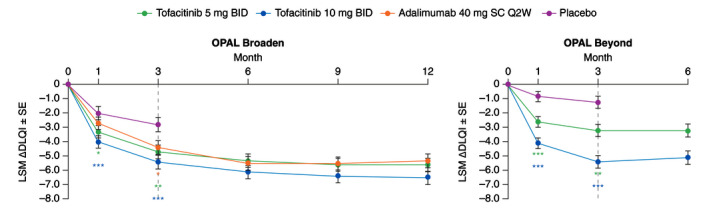
LSM (SE) change from baseline in DLQI total score (Prespecified analysis). *Nominal P ≤ 0.05; **P < 0.01; ***P < 0.001 vs. placebo. Dashed lines indicate the end of the placebo‐controlled period (Month 3). In the analyses of change from baseline in DLQI total score through the first 3 months, the two placebo‐to‐tofacitinib sequences were combined into a single placebo group (pooled placebo group); the results through Month 3 are from a repeated measures model. For results after Month 3, the two placebo‐to‐tofacitinib sequences were separate in another repeated measures model (data not shown). No imputation was applied for missing values. In OPAL Broaden, the numbers of unique patients included in the repeated measures model were 106, 104, 106 and 104 for tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg SC Q2W and placebo, respectively (same across all visits). In OPAL Beyond, the numbers of unique patients included in the repeated measures models were 128, 130 and 129 for tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo, respectively (same across all visits). ∆, change from baseline; BID, twice daily; DLQI, Dermatology Life Quality Index; LSM, least squares mean; Q2W, once every 2 weeks; SC, subcutaneous; SE, standard error.
